Ursodeoxycholic acid production by Gibberella zeae mutants
- PMID: 35939125
- PMCID: PMC9360310
- DOI: 10.1186/s13568-022-01446-2
Ursodeoxycholic acid production by Gibberella zeae mutants
Abstract
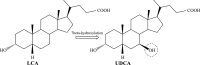
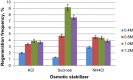
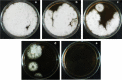
Ursodeoxycholic acid (UDCA) is a highly demanded pharmaceutical steroid widely used in medicine. An ascomycete Gibberella zeae VKM F-2600 is capable of producing UDCA by 7β-hydroxylation of lithocholic acid (LCA). The present study is aimed at the improvement of the fungus productivity. The original procedures for the protoplast obtaining followed by UV mutagenesis and screening of ketoconazole-resistant mutant clones have been applied. The highest yield of G. zeae protoplasts was obtained when using the mycelium in the active growth phase, ammonium chloride as an osmotic stabilizer and treatment of the fungal cells by the lytic enzymes cocktail from Trichoderma hurzanium. The conditions for effective protoplast regeneration and the UV-mutagenesis were found to provide 6-12% survival rate of the protoplasts with superior number of possible mutations. Three of 27 ketoconazole-resistant mutant clones obtained have been selected due to their increased biocatalytic activity towards LCA. The mutant G. zeae M23 produced 26% more UDCA even at relatively high LCA concentration (4 g/L) as compared with parent fungal strain, and the conversion reached 88% (w/w). The yield of UDCA reached in this study prefers those ever reported. The results contribute to the knowledge on ascomycete mutagenesis, and are of importance for biotechnological production of value added cholic acids.
Keywords: 7β-hydroxylation; Gibberella zeae; Lithocholic acid; Mutagenesis; Protoplasts; Ursodeoxycholic acid.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Biological synthesis of ursodeoxycholic acid.Front Microbiol. 2023 Feb 24;14:1140662. doi: 10.3389/fmicb.2023.1140662. eCollection 2023. Front Microbiol. 2023. PMID: 36910199 Free PMC article. Review.
-
Hydroxylation of lithocholic acid by selected actinobacteria and filamentous fungi.Steroids. 2013 Mar;78(3):370-8. doi: 10.1016/j.steroids.2012.12.010. Epub 2013 Jan 18. Steroids. 2013. PMID: 23333587
-
Comparative transcriptome analysis of the fungus Gibberella zeae transforming lithocholic acid into ursodeoxycholic acid.Biotechnol Lett. 2021 Feb;43(2):415-422. doi: 10.1007/s10529-020-03048-z. Epub 2020 Nov 11. Biotechnol Lett. 2021. PMID: 33179169
-
A fungal P450 enzyme from Fusarium equiseti HG18 with 7β-hydroxylase activity in biosynthesis of ursodeoxycholic acid.J Steroid Biochem Mol Biol. 2024 Jun;240:106507. doi: 10.1016/j.jsbmb.2024.106507. Epub 2024 Mar 18. J Steroid Biochem Mol Biol. 2024. PMID: 38508471
-
Regeneration and molecular characterization of an intergeneric hybrid between Graphium putredinis and Trichoderma harzianum by protoplasmic fusion.Biotechnol Adv. 2010 May-Jun;28(3):285-92. doi: 10.1016/j.biotechadv.2009.12.007. Epub 2010 Jan 11. Biotechnol Adv. 2010. PMID: 20064604 Review.
Cited by
-
Biological synthesis of ursodeoxycholic acid.Front Microbiol. 2023 Feb 24;14:1140662. doi: 10.3389/fmicb.2023.1140662. eCollection 2023. Front Microbiol. 2023. PMID: 36910199 Free PMC article. Review.
-
Improving Geldanamycin Production in Streptomyces geldanamycininus Through UV Mutagenesis of Protoplast.Microorganisms. 2025 Jan 17;13(1):186. doi: 10.3390/microorganisms13010186. Microorganisms. 2025. PMID: 39858954 Free PMC article.
-
PEG-Mediated Protoplast Transformation of Penicillium sclerotiorum (scaumcx01): Metabolomic Shifts and Root Colonization Dynamics.J Fungi (Basel). 2025 May 17;11(5):386. doi: 10.3390/jof11050386. J Fungi (Basel). 2025. PMID: 40422719 Free PMC article.
References
-
- Adams GC, Johnson N, Leslie JF, Hart LP. Heterokaryons of Gibberella zeae formed following hyphal anastomosis or protoplast fusion. Exp Mycol. 1987;11:339–353. doi: 10.1016/0147-5975(87)90022-3. - DOI
-
- Adams GC, Hart LP. The role of deoxynivalenol and 15-acetyldeoxynivalenol in pathogenesis by Gibberella zeae, as elucidated through protoplast fusions between toxigenic and nontoxigenic strains. Phytopathology. 1989;79:404–408. doi: 10.1094/Phyto-79-404. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

