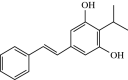
Tapinarof Cream 1%: First Approval
- PMID: 35939180
- PMCID: PMC9427914
- DOI: 10.1007/s40265-022-01748-6
Tapinarof Cream 1%: First Approval
Erratum in
-
Correction to: Tapinarof Cream 1%: First Approval.Drugs. 2022 Aug;82(12):1345. doi: 10.1007/s40265-022-01768-2. Drugs. 2022. PMID: 35976563 Free PMC article. No abstract available.
-
Correction: Tapinarof Cream 1%: First Approval.Drugs. 2022 Sep;82(13):1433. doi: 10.1007/s40265-022-01771-7. Drugs. 2022. PMID: 36040637 Free PMC article. No abstract available.
Abstract
Tapinarof cream 1% (VTAMA®) is an aryl hydrocarbon receptor (AhR) agonist that is being developed by Dermavant Sciences Inc. (a subsidiary of Roivant Sciences Inc.) as a once-daily topical treatment for plaque psoriasis and atopic dermatitis. AhR is a ligand-dependent transcription factor that has a role in immune-mediated skin responses. In May 2022, tapinarof cream 1% was approved in the USA for the topical treatment of plaque psoriasis in adults. Tapinarof cream 1% is also being investigated for the treatment of atopic dermatitis. This article summarizes the milestones in the development of tapinarof cream 1% leading to this first approval for the topical treatment of plaque psoriasis.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
Similar articles
-
Tapinarof Cream 1%: Pediatric First Approval.Paediatr Drugs. 2025 May;27(3):383-391. doi: 10.1007/s40272-025-00689-3. Paediatr Drugs. 2025. PMID: 40156765 Review.
-
Targeting the Aryl Hydrocarbon Receptor to Address the Challenges of Atopic Dermatitis.J Drugs Dermatol. 2024 Feb 1;23(2):23-28. doi: 10.36849/JDD.8026. J Drugs Dermatol. 2024. PMID: 38306128
-
Tapinarof, a Novel, First-in-Class, Topical Therapeutic Aryl Hydrocarbon Receptor Agonist for the Management of Psoriasis.J Drugs Dermatol. 2023 Aug 1;22(8):779-784. doi: 10.36849/jdd.7317. J Drugs Dermatol. 2023. PMID: 37556512
-
Characteristics and management of follicular events and contact dermatitis in patients using tapinarof cream for the treatment of atopic dermatitis or plaque psoriasis.J Dermatolog Treat. 2025 Dec;36(1):2517388. doi: 10.1080/09546634.2025.2517388. Epub 2025 Jul 2. J Dermatolog Treat. 2025. PMID: 40600584 Review.
-
VTAMA® (Tapinarof) Cream* for Plaque Psoriasis.Skinmed. 2022 Aug 31;20(4):298-300. eCollection 2022. Skinmed. 2022. PMID: 35976021
Cited by
-
Retinoic acid receptor structures: the journey from single domains to full-length complex.J Mol Endocrinol. 2022 Oct 11;69(4):T25-T36. doi: 10.1530/JME-22-0113. Print 2022 Nov 1. J Mol Endocrinol. 2022. PMID: 36069789 Free PMC article.
-
Phenotypic high-throughput screening identifies aryl hydrocarbon receptor agonism as common inhibitor of toxin-induced retinal pigment epithelium cell death.PLoS One. 2024 Apr 18;19(4):e0301239. doi: 10.1371/journal.pone.0301239. eCollection 2024. PLoS One. 2024. PMID: 38635505 Free PMC article.
-
A year in pharmacology: new drugs approved by the US Food and Drug Administration in 2022.Naunyn Schmiedebergs Arch Pharmacol. 2023 Aug;396(8):1619-1632. doi: 10.1007/s00210-023-02465-x. Epub 2023 Mar 23. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36951997 Free PMC article. Review.
-
Synthesis and clinical application of new drugs approved by FDA in 2022.Mol Biomed. 2023 Sep 4;4(1):26. doi: 10.1186/s43556-023-00138-y. Mol Biomed. 2023. PMID: 37661221 Free PMC article. Review.
-
FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis.Pharmaceutics. 2022 Nov 21;14(11):2538. doi: 10.3390/pharmaceutics14112538. Pharmaceutics. 2022. PMID: 36432728 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical