A Brain-Targeting Bispecific-Multivalent Antibody Clears Soluble Amyloid-Beta Aggregates in Alzheimer's Disease Mice
- PMID: 35939261
- PMCID: PMC9606191
- DOI: 10.1007/s13311-022-01283-y
A Brain-Targeting Bispecific-Multivalent Antibody Clears Soluble Amyloid-Beta Aggregates in Alzheimer's Disease Mice
Abstract
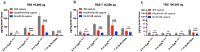
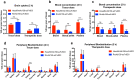
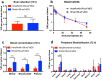
Amyloid-β (Aβ) oligomers and protofibrils are suggested to be the most neurotoxic Aβ species in Alzheimer's disease (AD). Hence, antibodies with strong and selective binding to these soluble Aβ aggregates are of therapeutic potential. We have recently introduced HexaRmAb158, a multivalent antibody with additional Aβ-binding sites in the form of single-chain fragment variables (scFv) on the N-terminal ends of Aβ protofibril selective antibody (RmAb158). Due to the additional binding sites and the short distance between them, HexaRmAb158 displayed a slow dissociation from protofibrils and strong binding to oligomers in vitro. In the current study, we aimed at investigating the therapeutic potential of this antibody format in vivo using mouse models of AD. To enhance BBB delivery, the transferrin receptor (TfR) binding moiety (scFv8D3) was added, forming the bispecific-multivalent antibody (HexaRmAb158-scFv8D3). The new antibody displayed a weaker TfR binding compared to the previously developed RmAb158-scFv8D3 and was less efficiently transcytosed in a cell-based BBB model. HexaRmAb158 detected soluble Aβ aggregates derived from brains of tg-ArcSwe and AppNL-G-F mice more efficiently compared to RmAb158. When intravenously injected, HexaRmAb158-scFv8D3 was actively transported over the BBB into the brain in vivo. Brain uptake was marginally lower than that of RmAb158-scFv8D3, but significantly higher than observed for conventional IgG antibodies. Both antibody formats displayed similar brain retention (72 h post injection) and equal capacity in clearing soluble Aβ aggregates in tg-ArcSwe mice. In conclusion, we demonstrate a bispecific-multivalent antibody format capable of passing the BBB and targeting a wide-range of sizes of soluble Aβ aggregates.
Keywords: Aβ; BBB; Bispecific; Mouse models; Multivalent antibodies; Oligomers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

