Structure of Human Enterovirus 70 and Its Inhibition by Capsid-Binding Compounds
- PMID: 35939401
- PMCID: PMC9472761
- DOI: 10.1128/jvi.00604-22
Structure of Human Enterovirus 70 and Its Inhibition by Capsid-Binding Compounds
Abstract
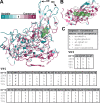
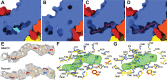
Enterovirus 70 (EV70) is a human pathogen belonging to the family Picornaviridae. EV70 is transmitted by eye secretions and causes acute hemorrhagic conjunctivitis, a serious eye disease. Despite the severity of the disease caused by EV70, its structure is unknown. Here, we present the structures of the EV70 virion, altered particle, and empty capsid determined by cryo-electron microscopy. The capsid of EV70 is composed of the subunits VP1, VP2, VP3, and VP4. The partially collapsed hydrophobic pocket located in VP1 of the EV70 virion is not occupied by a pocket factor, which is commonly present in other enteroviruses. Nevertheless, we show that the pocket can be targeted by the antiviral compounds WIN51711 and pleconaril, which block virus infection. The inhibitors prevent genome release by stabilizing EV70 particles. Knowledge of the structures of complexes of EV70 with inhibitors will enable the development of capsid-binding therapeutics against this virus. IMPORTANCE Globally distributed enterovirus 70 (EV70) causes local outbreaks of acute hemorrhagic conjunctivitis. The discharge from infected eyes enables the high-efficiency transmission of EV70 in overcrowded areas with low hygienic standards. Currently, only symptomatic treatments are available. We determined the structures of EV70 in its native form, the genome release intermediate, and the empty capsid resulting from genome release. Furthermore, we elucidated the structures of EV70 in complex with two inhibitors that block virus infection, and we describe the mechanism of their binding to the virus capsid. These results enable the development of therapeutics against EV70.
Keywords: Picornavirales; Picornaviridae; acute hemorrhagic conjunctivitis; antiviral; canyon; capsid; enterovirus; human; inhibitor; jelly roll; protein; structure; virion; virion structure; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Immunogenicity of enterovirus 70 capsid protein VP1 and its non-overlapping N- and C-terminal fragments.Antiviral Res. 2005 Jun;66(2-3):111-7. doi: 10.1016/j.antiviral.2005.02.004. Epub 2005 Mar 28. Antiviral Res. 2005. PMID: 15911028
-
Molecular basis for the acid-initiated uncoating of human enterovirus D68.Proc Natl Acad Sci U S A. 2018 Dec 26;115(52):E12209-E12217. doi: 10.1073/pnas.1803347115. Epub 2018 Dec 10. Proc Natl Acad Sci U S A. 2018. PMID: 30530701 Free PMC article.
-
A peptide vaccine based on a B-cell epitope on the VP1 protein of enterovirus 70 induces a strong antibody response.Acta Virol. 2012;56(4):337-42. doi: 10.4149/av_2012_04_337. Acta Virol. 2012. PMID: 23237090
-
[Fruit of the emergence of an enterovirus: acute haemorrhagic conjunctivitis].Ann Biol Clin (Paris). 2008 Sep-Oct;66(5):485-92. doi: 10.1684/abc.2008.0257. Ann Biol Clin (Paris). 2008. PMID: 18957336 Review. French.
-
[Enteroviruses responsible for acute hemorrhagic conjunctivitis].Med Mal Infect. 2010 Apr;40(4):212-8. doi: 10.1016/j.medmal.2009.09.006. Med Mal Infect. 2010. PMID: 19836177 Review. French.
Cited by
-
The early communication stages between serine proteases and enterovirus capsids in the race for viral disintegration.Commun Biol. 2024 Aug 9;7(1):969. doi: 10.1038/s42003-024-06627-2. Commun Biol. 2024. PMID: 39122806 Free PMC article.
-
Ocular Manifestations of Enterovirus: An Important Emerging Pathogen.Ophthalmol Sci. 2024 Jun 1;4(6):100562. doi: 10.1016/j.xops.2024.100562. eCollection 2024 Nov-Dec. Ophthalmol Sci. 2024. PMID: 39132021 Free PMC article. No abstract available.
-
Phylodynamic and Epistatic Analysis of Coxsackievirus A24 and Its Variant.Viruses. 2024 Aug 8;16(8):1267. doi: 10.3390/v16081267. Viruses. 2024. PMID: 39205241 Free PMC article.
-
Advances in the Treatment of Enterovirus-D68 and Rhinovirus Respiratory Infections.Infect Dis Rep. 2025 Jun 1;17(3):61. doi: 10.3390/idr17030061. Infect Dis Rep. 2025. PMID: 40559192 Free PMC article. Review.
-
Understanding the Neurotrophic Virus Mechanisms and Their Potential Effect on Systemic Lupus Erythematosus Development.Brain Sci. 2024 Jan 6;14(1):59. doi: 10.3390/brainsci14010059. Brain Sci. 2024. PMID: 38248274 Free PMC article. Review.
References
-
- Bruu A-L. 2002. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses, p 44–45. In Haaheim LR, Pattison JR, Whitley RJ (ed), A practical guide to clinical virology, 2nd ed. John Wiley & Sons Ltd, Chichester, United Kingdom.
-
- Shulman LM, Manor Y, Azar R, Handsher R, Vonsover A, Mendelson E, Rothman S, Hassin D, Halmut T, Abramovitz B, Varsano N. 1997. Identification of a new strain of fastidious enterovirus 70 as the causative agent of an outbreak of hemorrhagic conjunctivitis. J Clin Microbiol 35:2145–2149. 10.1128/jcm.35.8.2145-2149.1997. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

