Nitrite lowers the oxygen cost of ATP supply in cultured skeletal muscle cells by stimulating the rate of glycolytic ATP synthesis
- PMID: 35939418
- PMCID: PMC9359526
- DOI: 10.1371/journal.pone.0266905
Nitrite lowers the oxygen cost of ATP supply in cultured skeletal muscle cells by stimulating the rate of glycolytic ATP synthesis
Abstract
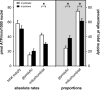
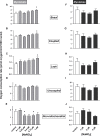
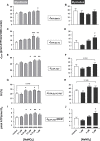
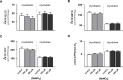
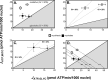
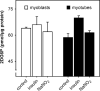
Dietary nitrate lowers the oxygen cost of human exercise. This effect has been suggested to result from stimulation of coupling efficiency of skeletal muscle oxidative phosphorylation by reduced nitrate derivatives. In this paper, we report the acute effects of sodium nitrite on the bioenergetic behaviour of cultured rat (L6) myocytes. At odds with improved efficiency of mitochondrial ATP synthesis, extracellular flux analysis reveals that a ½-hour exposure to NaNO2 (0.1-5 μM) does not affect mitochondrial coupling efficiency in static myoblasts or in spontaneously contracting myotubes. Unexpectedly, NaNO2 stimulates the rate of glycolytic ATP production in both myoblasts and myotubes. Increased ATP supply through glycolysis does not emerge at the expense of oxidative phosphorylation, which means that NaNO2 acutely increases the rate of overall myocellular ATP synthesis, significantly so in myoblasts and tending towards significance in contractile myotubes. Notably, NaNO2 exposure shifts myocytes to a more glycolytic bioenergetic phenotype. Mitochondrial oxygen consumption does not decrease after NaNO2 exposure, and non-mitochondrial respiration tends to drop. When total ATP synthesis rates are expressed in relation to total cellular oxygen consumption rates, it thus transpires that NaNO2 lowers the oxygen cost of ATP supply in cultured L6 myocytes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological .J Physiol. 2019 Nov;597(22):5429-5443. doi: 10.1113/JP278494. Epub 2019 Oct 30. J Physiol. 2019. PMID: 31541562 Free PMC article.
-
Treatment with Nitrate, but Not Nitrite, Lowers the Oxygen Cost of Exercise and Decreases Glycolytic Intermediates While Increasing Fatty Acid Metabolites in Exercised Zebrafish.J Nutr. 2019 Dec 1;149(12):2120-2132. doi: 10.1093/jn/nxz202. J Nutr. 2019. PMID: 31495890 Free PMC article.
-
Palmitate-induced changes in energy demand cause reallocation of ATP supply in rat and human skeletal muscle cells.Biochim Biophys Acta. 2016 Sep;1857(9):1403-1411. doi: 10.1016/j.bbabio.2016.04.286. Epub 2016 May 3. Biochim Biophys Acta. 2016. PMID: 27154056
-
Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation.J Exp Biol. 2001 Sep;204(Pt 18):3189-94. doi: 10.1242/jeb.204.18.3189. J Exp Biol. 2001. PMID: 11581333 Review.
-
Energy metabolism in muscle approaching maximal rates of oxygen utilization.Med Sci Sports Exerc. 1995 Jan;27(1):54-9. Med Sci Sports Exerc. 1995. PMID: 7898338 Review.
Cited by
-
Rapid identification of early renal damage in asymptomatic hyperuricemia patients based on urine Raman spectroscopy and bioinformatics analysis.Front Chem. 2023 Jan 25;11:1045697. doi: 10.3389/fchem.2023.1045697. eCollection 2023. Front Chem. 2023. PMID: 36762194 Free PMC article.
-
Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy.Nitric Oxide. 2023 Feb 1;131:8-17. doi: 10.1016/j.niox.2022.11.006. Epub 2022 Dec 5. Nitric Oxide. 2023. PMID: 36470373 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

