Improved efficiency for cross-arm comparisons via platform designs
- PMID: 35939566
- PMCID: PMC10583724
- DOI: 10.1093/biostatistics/kxac030
Improved efficiency for cross-arm comparisons via platform designs
Abstract
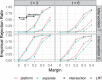
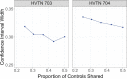
Though platform trials have been touted for their flexibility and streamlined use of trial resources, their statistical efficiency is not well understood. We fill this gap by establishing their greater efficiency for comparing the relative efficacy of multiple interventions over using several separate, 2-arm trials, where the relative efficacy of an arbitrary pair of interventions is evaluated by contrasting their relative risks as compared to control. In theoretical and numerical studies, we demonstrate that the inference of such a contrast using data from a platform trial enjoys identical or better precision than using data from separate trials, even when the former enrolls substantially fewer participants. This benefit is attributed to the sharing of controls among interventions under contemporaneous randomization. We further provide a novel procedure for establishing the noninferiority of a given intervention relative to the most efficacious of the other interventions under evaluation, where this procedure is adaptive in the sense that it need not be a priori known which of these other interventions is most efficacious. Our numerical studies show that this testing procedure can attain substantially better power when the data arise from a platform trial rather than multiple separate trials. Our results are illustrated using data from two monoclonal antibody trials for the prevention of HIV.
Keywords: COVID-19; Efficiency gain; Noninferiority test; Platform designs; Survival analysis; Vaccine efficacy.
© The Author 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Sep 6;22(1):592. doi: 10.1186/s13063-021-05484-2. Trials. 2021. PMID: 34488843 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Psychological interventions for needle-related procedural pain and distress in children and adolescents.Cochrane Database Syst Rev. 2018 Oct 4;10(10):CD005179. doi: 10.1002/14651858.CD005179.pub4. Cochrane Database Syst Rev. 2018. PMID: 30284240 Free PMC article.
-
Interventions for supporting the initiation and continuation of breastfeeding among women who are overweight or obese.Cochrane Database Syst Rev. 2019 Sep 17;9(9):CD012099. doi: 10.1002/14651858.CD012099.pub2. Cochrane Database Syst Rev. 2019. PMID: 31529625 Free PMC article.
Cited by
-
The impact of heterogeneity on the analysis of platform trials with normally distributed outcomes.BMC Med Res Methodol. 2024 Jul 30;24(1):163. doi: 10.1186/s12874-024-02293-4. BMC Med Res Methodol. 2024. PMID: 39080538 Free PMC article.
-
Current state-of-the-art and gaps in platform trials: 10 things you should know, insights from EU-PEARL.EClinicalMedicine. 2023 Dec 26;67:102384. doi: 10.1016/j.eclinm.2023.102384. eCollection 2024 Jan. EClinicalMedicine. 2023. PMID: 38226342 Free PMC article. Review.
References
-
- Choko, A., Fielding, K., Stallard, N., Maheswaran, H., Lepine, A., Desmond, N., Kumwenda, M. and Corbett, E. (2017). Investigating interventions to increase uptake of HIV testing and linkage into care or prevention for male partners of pregnant women in antenatal clinics in Blantyre, Malawi: study protocol for a cluster randomised trial. Trials 18, 349. - PMC - PubMed
-
- D’Agostino, R., Massaro, J. and Sullivan, L. (2003). Non-inferiority trials: design concepts and issues — the encounters of academic consultants in statistics. Statistics in Medicine 22, 169–186. - PubMed
-
- Everson-Stewart, S. and Emerson, S. (2010). Bio-creep in non-inferiority clinical trials. Statistics in Medicine 29, 2769–2780. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

