Reconstitution of the S. aureus agr quorum sensing pathway reveals a direct role for the integral membrane protease MroQ in pheromone biosynthesis
- PMID: 35939668
- PMCID: PMC9388083
- DOI: 10.1073/pnas.2202661119
Reconstitution of the S. aureus agr quorum sensing pathway reveals a direct role for the integral membrane protease MroQ in pheromone biosynthesis
Abstract
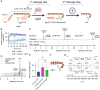
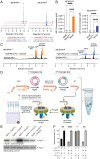
In Staphylococcus aureus, virulence is under the control of a quorum sensing (QS) circuit encoded in the accessory gene regulator (agr) genomic locus. Key to this pathogenic behavior is the production and signaling activity of a secreted pheromone, the autoinducing peptide (AIP), generated following the ribosomal synthesis and posttranslational modification of a precursor polypeptide, AgrD, through two discrete cleavage steps. The integral membrane protease AgrB is known to catalyze the first processing event, generating the AIP biosynthetic intermediate, AgrD (1-32) thiolactone. However, the identity of the second protease in this biosynthetic pathway, which removes an N-terminal leader sequence, has remained ambiguous. Here, we show that membrane protease regulator of agr QS (MroQ), an integral membrane protease recently implicated in the agr response, is directly involved in AIP production. Genetic complementation and biochemical experiments reveal that MroQ proteolytic activity is required for AIP biosynthesis in agr specificity group I and group II, but not group III. Notably, as part of this effort, the biosynthesis and AIP-sensing arms of the QS circuit were reconstituted together in vitro. Our experiments also reveal the molecular features guiding MroQ cleavage activity, a critical factor in defining agr specificity group identity. Collectively, our study adds to the molecular understanding of the agr response and Staphylococcus aureus virulence.
Keywords: RiPP biosynthesis; Staphylococcus aureus; bacterial pathogenesis; quorum sensing; synthetic biology.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Determinants of maturation of the Staphylococcus aureus autoinducing peptide.J Bacteriol. 2024 Sep 19;206(9):e0019524. doi: 10.1128/jb.00195-24. Epub 2024 Aug 23. J Bacteriol. 2024. PMID: 39177535 Free PMC article.
-
Characterization of MroQ-Dependent Maturation and Export of the Staphylococcus aureus Accessory Gene Regulatory System Autoinducing Peptide.Infect Immun. 2022 Oct 20;90(10):e0026322. doi: 10.1128/iai.00263-22. Epub 2022 Sep 8. Infect Immun. 2022. PMID: 36073934 Free PMC article.
-
Control of Staphylococcus aureus Quorum Sensing by a Membrane-Embedded Peptidase.Infect Immun. 2019 Apr 23;87(5):e00019-19. doi: 10.1128/IAI.00019-19. Print 2019 Mar. Infect Immun. 2019. PMID: 30833334 Free PMC article.
-
What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia?Trends Microbiol. 2014 Dec;22(12):676-85. doi: 10.1016/j.tim.2014.09.002. Epub 2014 Oct 6. Trends Microbiol. 2014. PMID: 25300477 Review.
-
Quorum-sensing, intra- and inter-species competition in the staphylococci.Microbiology (Reading). 2023 Aug;169(8):001381. doi: 10.1099/mic.0.001381. Microbiology (Reading). 2023. PMID: 37578829 Free PMC article. Review.
Cited by
-
Chemical and biomolecular insights into the Staphylococcus aureus agr quorum sensing system: Current progress and ongoing challenges.Isr J Chem. 2023 Jun;63(5-6):e202200096. doi: 10.1002/ijch.202200096. Epub 2023 Mar 16. Isr J Chem. 2023. PMID: 38765792 Free PMC article.
-
Modelled-Microgravity Reduces Virulence Factor Production in Staphylococcus aureus through Downregulation of agr-Dependent Quorum Sensing.Int J Mol Sci. 2023 Nov 6;24(21):15997. doi: 10.3390/ijms242115997. Int J Mol Sci. 2023. PMID: 37958979 Free PMC article.
-
Toxic Shock Syndrome Toxin-1 (TSST-1) in Staphylococcus aureus: Prevalence, Molecular Mechanisms, and Public Health Implications.Toxins (Basel). 2025 Jun 24;17(7):323. doi: 10.3390/toxins17070323. Toxins (Basel). 2025. PMID: 40711134 Free PMC article. Review.
-
Characterization of an Autoinducing Peptide Signal Reveals Highly Efficacious Synthetic Inhibitors and Activators of Quorum Sensing and Biofilm Formation in Listeria monocytogenes.Biochemistry. 2023 Oct 3;62(19):2878-2892. doi: 10.1021/acs.biochem.3c00373. Epub 2023 Sep 12. Biochemistry. 2023. PMID: 37699554 Free PMC article.
-
Proteases Involved in Leader Peptide Removal during RiPP Biosynthesis.ACS Bio Med Chem Au. 2023 Dec 13;4(1):20-36. doi: 10.1021/acsbiomedchemau.3c00059. eCollection 2024 Feb 21. ACS Bio Med Chem Au. 2023. PMID: 38404746 Free PMC article. Review.
References
-
- Miller M. B., Bassler B. L., Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199 (2001). - PubMed
-
- Novick R. P., Geisinger E., Quorum sensing in staphylococci. Annu. Rev. Genet. 42, 541–564 (2008). - PubMed
-
- Thompson R. L., Cabezudo I., Wenzel R. P., Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 97, 309–317 (1982). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

