Lactobacillus crispatus Limits Bladder Uropathogenic E. coli Infection by Triggering a Host Type I Interferon Response
- PMID: 35939684
- PMCID: PMC9388105
- DOI: 10.1073/pnas.2117904119
Lactobacillus crispatus Limits Bladder Uropathogenic E. coli Infection by Triggering a Host Type I Interferon Response
Abstract
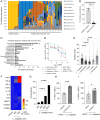
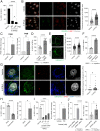
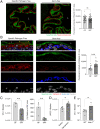
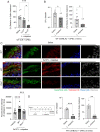
Many urinary tract infections (UTIs) are recurrent because uropathogens persist within the bladder epithelial cells (BECs) for extended periods between bouts of infection. Because persistent uropathogens are intracellular, they are often refractive to antibiotic treatment. The recent discovery of endogenous Lactobacillus spp. in the bladders of healthy humans raised the question of whether these endogenous bacteria directly or indirectly impact intracellular bacterial burden in the bladder. Here, we report that in contrast to healthy women, female patients experiencing recurrent UTIs have a bladder population of Lactobacilli that is markedly reduced. Exposing infected human BECs to L. crispatus in vitro markedly reduced the intracellular uropathogenic Escherichia coli (UPEC) load. The adherence of Lactobacilli to BECs was found to result in increased type I interferon (IFN) production, which in turn enhanced the expression of cathepsin D within lysosomes harboring UPECs. This lysosomal cathepsin D-mediated UPEC killing was diminished in germ-free mice and type I IFN receptor-deficient mice. Secreted metabolites of L. crispatus seemed to be responsible for the increased expression of type I IFN in human BECs. Intravesicular administration of Lactobacilli into UPEC-infected murine bladders markedly reduced their intracellular bacterial load suggesting that components of the endogenous microflora can have therapeutic effects against UTIs.
Keywords: Lactobacilli; commensal bacteria; type I interferon; urinary tract infection.
Conflict of interest statement
The authors declare no competing interest.
Figures
Comment in
-
Uro-Science.J Urol. 2023 Apr;209(4):801-802. doi: 10.1097/JU.0000000000003161. Epub 2023 Jan 19. J Urol. 2023. PMID: 36655472 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

