Structural basis of higher order oligomerization of KSHV inhibitor of cGAS
- PMID: 35939686
- PMCID: PMC9388135
- DOI: 10.1073/pnas.2200285119
Structural basis of higher order oligomerization of KSHV inhibitor of cGAS
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) inhibitor of cyclic GMP-AMP synthase (cGAS) (KicGAS) encoded by ORF52 is a conserved major tegument protein of KSHV and the first reported viral inhibitor of cGAS. In our previous study, we found that KicGAS is highly oligomerized in solution and that oligomerization is required for its cooperative DNA binding and for inhibiting DNA-induced phase separation and activation of cGAS. However, how KicGAS oligomerizes remained unclear. Here, we present the crystal structure of KicGAS at 2.5 Å resolution, which reveals an "L"-shaped molecule with each arm of the L essentially formed by a single α helix (α1 and α2). Antiparallel dimerization of α2 helices from two KicGAS molecules leads to a unique "Z"-shaped dimer. Surprisingly, α1 is also a dimerization domain. It forms a parallel dimeric leucine zipper with the α1 from a neighboring dimer, leading to the formation of an infinite chain of KicGAS dimers. Residues involved in leucine zipper dimer formation are among the most conserved residues across ORF52 homologs of gammaherpesviruses. The self-oligomerization increases the valence and cooperativity of interaction with DNA. The resultant multivalent interaction is critical for the formation of liquid condensates with DNA and consequent sequestration of DNA from being sensed by cGAS, explaining its role in restricting cGAS activation. The structure presented here not only provides a mechanistic understanding of the function of KicGAS but also informs a molecular target for rational design of antivirals against KSHV and related viruses.
Keywords: DNA binding; KicGAS; ORF52; cGAS; higher order oligomerization.
Conflict of interest statement
The authors declare no competing interest.
Figures
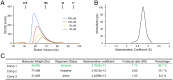


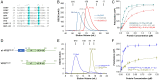

References
-
- Hopfner K.-P., Hornung V., Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI146330/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 DE026101/DE/NIDCR NIH HHS/United States
- R01DE026101/HHS | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- R01 AI146330/AI/NIAID NIH HHS/United States
- R00AI108793/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources

