Oropouche orthobunyavirus infection is mediated by the cellular host factor Lrp1
- PMID: 35939689
- PMCID: PMC9388146
- DOI: 10.1073/pnas.2204706119
Oropouche orthobunyavirus infection is mediated by the cellular host factor Lrp1
Abstract
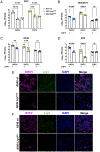
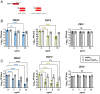
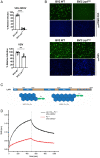
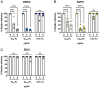
Oropouche orthobunyavirus (OROV; Peribunyaviridae) is a mosquito-transmitted virus that causes widespread human febrile illness in South America, with occasional progression to neurologic effects. Host factors mediating the cellular entry of OROV are undefined. Here, we show that OROV uses the host protein low-density lipoprotein-related protein 1 (Lrp1) for efficient cellular infection. Cells from evolutionarily distinct species lacking Lrp1 were less permissive to OROV infection than cells with Lrp1. Treatment of cells with either the high-affinity Lrp1 ligand receptor-associated protein (RAP) or recombinant ectodomain truncations of Lrp1 significantly reduced OROV infection. In addition, chimeric vesicular stomatitis virus (VSV) expressing OROV glycoproteins (VSV-OROV) bound to the Lrp1 ectodomain in vitro. Furthermore, we demonstrate the biological relevance of the OROV-Lrp1 interaction in a proof-of-concept mouse study in which treatment of mice with RAP at the time of infection reduced tissue viral load and promoted survival from an otherwise lethal infection. These results with OROV, along with the recent finding of Lrp1 as an entry factor for Rift Valley fever virus, highlight the broader significance of Lrp1 in cellular infection by diverse bunyaviruses. Shared strategies for entry, such as the critical function of Lrp1 defined here, provide a foundation for the development of pan-bunyaviral therapeutics.
Keywords: Lrp1; Oropouche virus; bunyavirus; entry factor; lipoprotein.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Barr J. N., Weber F., Schmaljohn C. S., “Bunyavirales: The viruses and their replication” in Fields Virology Volume 1: Emerging Viruses, Knipe D. M., Howley P., Whelan S. P., Eds. (Wolters Kluwer, Philadelphia, 2020), chap. 16. pp 707–749.
-
- McMullan L. K., et al. , A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367, 834–841 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

