Muscle degeneration in chronic massive rotator cuff tears of the shoulder: Addressing the real problem using a graphene matrix
- PMID: 35939692
- PMCID: PMC9388153
- DOI: 10.1073/pnas.2208106119
Muscle degeneration in chronic massive rotator cuff tears of the shoulder: Addressing the real problem using a graphene matrix
Abstract
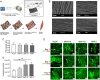
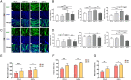
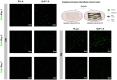
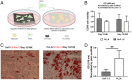
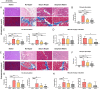
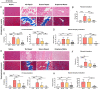
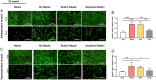
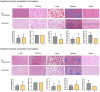
Massive rotator cuff tears (MRCTs) of the shoulder cause disability and pain among the adult population. In chronic injuries, the tendon retraction and subsequently the loss of mechanical load lead to muscle atrophy, fat accumulation, and fibrosis formation over time. The intrinsic repair mechanism of muscle and the successful repair of the torn tendon cannot reverse the muscle degeneration following MRCTs. To address these limitations, we developed an electroconductive matrix by incorporating graphene nanoplatelets (GnPs) into aligned poly(l-lactic acid) (PLLA) nanofibers. This study aimed to understand 1) the effects of GnP matrices on muscle regeneration and inhibition of fat formation in vitro and 2) the ability of GnP matrices to reverse muscle degenerative changes in vivo following an MRCT. The GnP matrix significantly increased myotube formation, which can be attributed to enhanced intracellular calcium ions in myoblasts. Moreover, the GnP matrix suppressed adipogenesis in adipose-derived stem cells. These results supported the clinical effects of the GnP matrix on reducing fat accumulation and muscle atrophy. The histological evaluation showed the potential of the GnP matrix to reverse muscle atrophy, fat accumulation, and fibrosis in both supraspinatus and infraspinatus muscles at 24 and 32 wk after the chronic MRCTs of the rat shoulder. The pathological evaluation of internal organs confirmed the long-term biocompatibility of the GnP matrix. We found that reversing muscle degenerative changes improved the morphology and tensile properties of the tendon compared with current surgical techniques. The long-term biocompatibility and the ability of the GnP matrix to treat muscle degeneration are promising for the realization of MRCT healing and regeneration.
Keywords: fat accumulation; graphene; muscle degeneration; rotator cuff tears.
Conflict of interest statement
Competing interest statement: University of Connecticut has filed a patent entitled “Graphene-Based Nanofibers for Skeletal Muscle Tissue Regeneration” on behalf of the inventors, N.S.S. and C.T.L. C.T.L. has the following competing financial interests: Biorez, Globus, HOT, HOT Bone, Kuros Bioscience, NPD, and Cobb (W. Montague) NMA Health Institute. L.S.N. has the following competing financial interests: Biorez.
Figures

Similar articles
-
Efficacy of a Novel Electroconductive Matrix To Treat Muscle Atrophy and Fat Accumulation in Chronic Massive Rotator Cuff Tears of the Shoulder.ACS Biomater Sci Eng. 2023 Oct 9;9(10):5782-5792. doi: 10.1021/acsbiomaterials.3c00585. Epub 2023 Sep 28. ACS Biomater Sci Eng. 2023. PMID: 37769114
-
Exosomes Isolated From Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs.Am J Sports Med. 2019 Nov;47(13):3247-3255. doi: 10.1177/0363546519876323. Epub 2019 Sep 27. Am J Sports Med. 2019. PMID: 31560856
-
Beige fibro-adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears.J Shoulder Elbow Surg. 2020 Apr;29(4):719-727. doi: 10.1016/j.jse.2019.09.021. Epub 2019 Nov 26. J Shoulder Elbow Surg. 2020. PMID: 31784382 Free PMC article.
-
Cellular and molecular modulation of rotator cuff muscle pathophysiology.J Orthop Res. 2021 Nov;39(11):2310-2322. doi: 10.1002/jor.25179. Epub 2021 Sep 30. J Orthop Res. 2021. PMID: 34553789 Review.
-
Rotator cuff tear degeneration and the role of fibro-adipogenic progenitors.Ann N Y Acad Sci. 2021 Apr;1490(1):13-28. doi: 10.1111/nyas.14437. Epub 2020 Jul 29. Ann N Y Acad Sci. 2021. PMID: 32725671 Free PMC article. Review.
Cited by
-
Studying intramuscular fat deposition and muscle regeneration: insights from a comparative analysis of mouse strains, injury models, and sex differences.Skelet Muscle. 2024 May 29;14(1):12. doi: 10.1186/s13395-024-00344-4. Skelet Muscle. 2024. PMID: 38812056 Free PMC article.
-
PIEZO1 activation enhances myogenesis and mitigates muscle degeneration in rotator cuff tear.Regen Ther. 2024 Dec 13;28:143-152. doi: 10.1016/j.reth.2024.12.002. eCollection 2025 Mar. Regen Ther. 2024. PMID: 39759799 Free PMC article.
-
Classes of Stem Cells: From Biology to Engineering.Regen Eng Transl Med. 2024 Sep;10(3):309-322. doi: 10.1007/s40883-023-00317-x. Epub 2023 Sep 18. Regen Eng Transl Med. 2024. PMID: 39387056 Free PMC article.
-
Targeting RAGE-signaling pathways in the repair of rotator-cuff injury.Mol Cell Biochem. 2025 Apr;480(4):2539-2554. doi: 10.1007/s11010-024-05132-8. Epub 2024 Oct 12. Mol Cell Biochem. 2025. PMID: 39395136 Free PMC article. Review.
-
Interposition Grafting Using Fascia Lata Autograft for Failed Rotator Cuff Repairs.Arthrosc Tech. 2023 Dec 18;13(1):102822. doi: 10.1016/j.eats.2023.08.027. eCollection 2024 Jan. Arthrosc Tech. 2023. PMID: 38312872 Free PMC article.
References
-
- Hsu J. E., Horneff J. G., Gee A. O., Immobilization after rotator cuff repair: What evidence do we have now? Orthop. Clin. North Am. 47, 169–177 (2016). - PubMed
-
- Novakova S. S., et al. , Tissue-engineered tendon constructs for rotator cuff repair in sheep. J. Orthop. Res. 36, 289–299 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

