Surgical outcomes in people with hemophilia A taking emicizumab prophylaxis: experience from the HAVEN 1-4 studies
- PMID: 35939785
- PMCID: PMC9768240
- DOI: 10.1182/bloodadvances.2022007458
Surgical outcomes in people with hemophilia A taking emicizumab prophylaxis: experience from the HAVEN 1-4 studies
Abstract
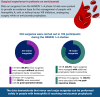
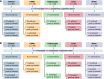
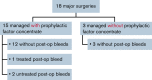
Many people with hemophilia A (PwHA) undergo surgery in their lifetime, often because of complications of their disease. Emicizumab is the first bispecific monoclonal antibody prophylactic therapy for PwHA, and its efficacy and safety have been previously demonstrated; however, there is a need to build an evidence base on the management of PwHA on emicizumab undergoing surgery. Data from the HAVEN 1-4 phase 3 clinical trials were pooled to provide a summary of all minor and major surgeries in PwHA with or without factor VIII (FVIII) inhibitors who were receiving emicizumab prophylaxis. Overall, 233 surgeries were carried out during the HAVEN 1-4 trials: 215 minor surgeries (including minor dental and joint procedures, central venous access device placement or removal, and endoscopies) in 115 PwHA (64 with FVIII inhibitors) and 18 major surgeries (including arthroplasty and synovectomy) in 18 PwHA (10 with FVIII inhibitors). Perioperative hemostatic support was at the discretion of the treating physician. Overall, the median (interquartile range [IQR]) age was 33.5 (13.0-49.0) years and the median (IQR) emicizumab exposure time before surgery was 278.0 (177.0-431.0) days. Among the 215 minor surgeries, 141 (65.6%) were managed without additional prophylactic factor concentrate, and of those, 121 (85.8%) were not associated with a postoperative bleed. The majority (15 of 18 [83.3%]) of major surgeries were managed with additional prophylactic factor concentrate. Twelve (80.0%) of these 15 surgeries were associated with no intraoperative or postoperative bleeds. The data demonstrate that minor and major surgeries can be performed safely in PwHA receiving emicizumab prophylaxis. These trials are registered at www.clinicaltrials.gov as #NCT02622321, #NCT02795767, #NCT02847637, and #NCT03020160.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.K.-J. has received honoraria and consultancy fees from Genentech, Inc./F. Hoffmann-La Roche Ltd., CSL Behring, BioMarin, and CRISPR; has been a member of the speaker’s bureau for Genentech, Inc./F. Hoffmann-La Roche Ltd., and Sanofi; and has received research funding from Genentech, Inc. F.P. has received speaker fees for participating in educational symposia and advisory boards for F. Hoffmann-La Roche Ltd., Sanofi, SOBI, and Takeda. J.O. has received personal/consultancy fees for travel support, advisory boards, and symposia from Bayer, Biogen Idec, BioMarin, Biotest, Chugai Pharmaceutical Co., Ltd., CSL Behring, Freeline, Grifols, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd., Sanofi, Spark Therapeutics, SOBI, and Shire/Takeda; and grants from Bayer, Biotest, CSL Behring, Octapharma, and Pfizer. T.C. is an employee of Spark Therapeutics and a former employee of Genentech, Inc. S.C. is an employee and holds stocks in F. Hoffmann-La Roche Ltd. M.Y.D. is an employee of Genentech, Inc. and holds stocks in F. Hoffmann-La Roche Ltd. S.E.C. has received consultancy fees for Bayer, BioMarin, CSL Behring, HEMA Biologics, Pfizer, and Sanofi; research funding from F. Hoffmann-La Roche Ltd./Genentech, Inc.; and is a member on another entity’s Board of Directors or its advisory committees for Hemophilia Alliance and American Thrombosis and Hemostasis Network. T.L. has served as a consultant for CSL Behring, F. Hoffmann-La Roche Ltd., and SOBI. C.L.K. has received honoraria for participation in advisory boards for Sanofi US, Takeda, and Spark Therapeutics. S.W.P. has served as a consultant to Apcintex, ASC Therapeutics, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, GenVentiv, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, F. Hoffmann-La Roche Ltd./Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, and UniQure. R.H.K. and B.T. are employees and hold stock in Genentech, Inc. C.D., N.S.B., and M.N. are employees of F. Hoffmann-La Roche Ltd. M.L. is an employee and holds stocks in F. Hoffmann-La Roche Ltd. I.P.-P. is a former employee of Genentech, Inc. G.Y. reports research funding from Genentech, Inc., Grifols, and Takeda; paid testimony from CSL Behring and Genentech, Inc.; consultancy fees from Apcintex, Bayer, BioMarin, Genentech, Inc./F. Hoffmann-La Roche, Novo Nordisk, Pfizer, Sanofi/Genzyme, Spark Therapeutics, and Takeda; and honoraria from Genentech, Inc., Sanofi, and Spark Therapeutics. V.J.-Y. has received reimbursement for attending symposia/congresses, honoraria for speaking, consulting and/or research funding from Takeda, Bayer, CSL Behring, Grifols, Novo Nordisk, SOBI, F. Hoffmann-La Roche Ltd., Octapharma, BioMarin, Sanofi, and Pfizer.
The current affiliation for T.C. is Spark Therapeutics, Inc., Philadelphia, PA.
The current affiliation for I.P.-P. is Graphite Bio, Inc., South San Francisco, CA.
Figures

References
-
- Escobar M, Maahs J, Hellman E, et al. Multidisciplinary management of patients with haemophilia with inhibitors undergoing surgery in the United States: perspectives and best practices derived from experienced treatment centres. Haemophilia. 2012;18(6):971–981. - PubMed
-
- Jiménez-Yuste V, Rodriguez-Merchan EC, Alvarez MT, Quintana M, Fernandez I, Hernandez-Navarro F. Controversies and challenges in elective orthopedic surgery in patients with hemophilia and inhibitors. Semin Hematol. 2008;45(2 suppl 1):S64–S67. - PubMed

