Standardization of Synthetic Biology Tools and Assembly Methods for Saccharomyces cerevisiae and Emerging Yeast Species
- PMID: 35939789
- PMCID: PMC9396660
- DOI: 10.1021/acssynbio.1c00442
Standardization of Synthetic Biology Tools and Assembly Methods for Saccharomyces cerevisiae and Emerging Yeast Species
Abstract
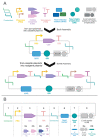
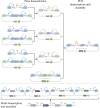
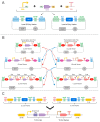
As redesigning organisms using engineering principles is one of the purposes of synthetic biology (SynBio), the standardization of experimental methods and DNA parts is becoming increasingly a necessity. The synthetic biology community focusing on the engineering of Saccharomyces cerevisiae has been in the foreground in this area, conceiving several well-characterized SynBio toolkits widely adopted by the community. In this review, the molecular methods and toolkits developed for S. cerevisiae are discussed in terms of their contributions to the required standardization efforts. In addition, the toolkits designed for emerging nonconventional yeast species including Yarrowia lipolytica, Komagataella phaffii, and Kluyveromyces marxianus are also reviewed. Without a doubt, the characterized DNA parts combined with the standardized assembly strategies highlighted in these toolkits have greatly contributed to the rapid development of many metabolic engineering and diagnostics applications among others. Despite the growing capacity in deploying synthetic biology for common yeast genome engineering works, the yeast community has a long journey to go to exploit it in more sophisticated and delicate applications like bioautomation.
Keywords: automation; biological parts; characterization; standardization; synthetic biology; yeast toolkits.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Recent advances in construction and regulation of yeast cell factories.World J Microbiol Biotechnol. 2022 Feb 17;38(4):57. doi: 10.1007/s11274-022-03241-4. World J Microbiol Biotechnol. 2022. PMID: 35174424 Review.
-
Synthetic Biology Expands the Industrial Potential of Yarrowia lipolytica.Trends Biotechnol. 2018 Oct;36(10):1085-1095. doi: 10.1016/j.tibtech.2018.05.004. Epub 2018 Jun 4. Trends Biotechnol. 2018. PMID: 29880228 Review.
-
Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts.Biotechnol Adv. 2021 Mar-Apr;47:107695. doi: 10.1016/j.biotechadv.2021.107695. Epub 2021 Jan 16. Biotechnol Adv. 2021. PMID: 33465474 Review.
-
Metabolic Engineering of Yarrowia lipolytica for Terpenoid Production: Tools and Strategies.ACS Synth Biol. 2023 Mar 17;12(3):639-656. doi: 10.1021/acssynbio.2c00569. Epub 2023 Mar 3. ACS Synth Biol. 2023. PMID: 36867718 Review.
-
Yeast synthetic biology for the production of recombinant therapeutic proteins.FEMS Yeast Res. 2015 Feb;15(1):1-16. doi: 10.1111/1567-1364.12195. Epub 2015 Jan 14. FEMS Yeast Res. 2015. PMID: 25130199
Cited by
-
Improved production of Taxol® precursors in S. cerevisiae using combinatorial in silico design and metabolic engineering.Microb Cell Fact. 2023 Nov 29;22(1):243. doi: 10.1186/s12934-023-02251-7. Microb Cell Fact. 2023. PMID: 38031061 Free PMC article.
-
Automated in vivo enzyme engineering accelerates biocatalyst optimization.Nat Commun. 2024 Apr 24;15(1):3447. doi: 10.1038/s41467-024-46574-4. Nat Commun. 2024. PMID: 38658554 Free PMC article. Review.
-
ACtivE: Assembly and CRISPR-Targeted in Vivo Editing for Yeast Genome Engineering Using Minimum Reagents and Time.ACS Synth Biol. 2022 Nov 18;11(11):3629-3643. doi: 10.1021/acssynbio.2c00175. Epub 2022 Oct 17. ACS Synth Biol. 2022. PMID: 36252276 Free PMC article.
-
AutoBioTech─A Versatile Biofoundry for Automated Strain Engineering.ACS Synth Biol. 2024 Jul 19;13(7):2227-2237. doi: 10.1021/acssynbio.4c00298. Epub 2024 Jul 8. ACS Synth Biol. 2024. PMID: 38975718 Free PMC article.
-
Characterizing a standardized BioPart for PVQ-specific expression in C. elegans.MicroPubl Biol. 2023 Jun 22;2023:10.17912/micropub.biology.000870. doi: 10.17912/micropub.biology.000870. eCollection 2023. MicroPubl Biol. 2023. PMID: 37426742 Free PMC article.
References
-
- Xie Z.; Hall J.; McCarthy I. P.; Skitmore M.; Shen L. Standardization Efforts: The Relationship between Knowledge Dimensions, Search Processes and Innovation Outcomes. Technovation 2016, 48–49, 69–78. 10.1016/j.technovation.2015.12.002. - DOI
-
- Saltzman J.; Chatterjee S.; Raman M. A Framework for ICT Standards Creation: The Case of ITU-T Standard H.350. Inf. Syst. 2008, 33 (3), 285–299. 10.1016/j.is.2007.10.001. - DOI
-
- Khan A. S.; Karim A. Importance of Standards in Engineering and Technology Education. Int. J. Educ. Pedagog. Sci. 2016, 10 (3), 1050–1054. 10.5281/ZENODO.1127348. - DOI
-
- Russell A. L. Standardization in History: A Review Essay with an Eye to the Future. In The Standards Edge: Future Generation; The Bolin Group, 2005.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

