Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms
- PMID: 35939855
- PMCID: PMC9552261
- DOI: 10.1016/j.bioorg.2022.106070
Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms
Abstract
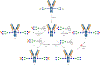
Sulfation is a common modification of glycans and glycoproteins. Sulfated N-glycans have been identified in various glycoproteins and implicated for biological functions, but in vitro synthesis of structurally well-defined full length sulfated N-glycans remains to be described. We report here the first in vitro enzymatic sulfation of biantennary complex type N-glycans by recombinant human CHST2 (GlcNAc-6-O-sulfotransferase 1, GlcNAc6ST-1). We found that the sulfotransferase showed high antennary preference and could selectively sulfate the GlcNAc moiety located on the Manα1,3Man arm of the biantennary N-glycan. The glycan chain was further elongated by bacterial β1,4 galactosyltransferase from Neiserria meningitidis and human β1,4 galactosyltransferase IV(B4GALT4), which led to the formation of different sulfated N-glycans. Using rituximab as a model IgG antibody, we further demonstrated that the sulfated N-glycans could be efficiently transferred to an intact antibody by using a chemoenzymatic Fc glycan remodeling method, providing homogeneous sulfated glycoforms of antibodies. Preliminary binding analysis indicated that sulfation did not affect the apparent affinity of the antibody for FcγIIIa receptor.
Keywords: Chemoenzymatic synthesis; Glycoforms; Sulfated N-glycans; Sulfated antibody; Sulfation; Sulfotransferase.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


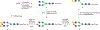
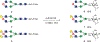
Similar articles
-
Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins.JACS Au. 2024 Jul 26;4(8):2966-2978. doi: 10.1021/jacsau.4c00307. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211606 Free PMC article.
-
Installation of O-glycan sulfation capacities in human HEK293 cells for display of sulfated mucins.J Biol Chem. 2022 Feb;298(2):101382. doi: 10.1016/j.jbc.2021.101382. Epub 2021 Dec 24. J Biol Chem. 2022. PMID: 34954141 Free PMC article.
-
GlcNAc6ST-1 regulates sulfation of N-glycans and myelination in the peripheral nervous system.Sci Rep. 2017 Feb 10;7:42257. doi: 10.1038/srep42257. Sci Rep. 2017. PMID: 28186137 Free PMC article.
-
Roles of sulfated glycans in lymphocyte homing.Biol Pharm Bull. 2006 Dec;29(12):2343-9. doi: 10.1248/bpb.29.2343. Biol Pharm Bull. 2006. PMID: 17142960 Review.
-
Phylogeny, structure, function, biosynthesis and evolution of sulfated galactose-containing glycans.Int J Biol Macromol. 2016 Mar;84:372-9. doi: 10.1016/j.ijbiomac.2015.12.035. Epub 2015 Dec 19. Int J Biol Macromol. 2016. PMID: 26712697 Review.
Cited by
-
Milk glycan metabolism by intestinal bifidobacteria: insights from comparative genomics.Crit Rev Biochem Mol Biol. 2022 Oct-Dec;57(5-6):562-584. doi: 10.1080/10409238.2023.2182272. Epub 2023 Mar 3. Crit Rev Biochem Mol Biol. 2022. PMID: 36866565 Free PMC article. Review.
-
Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins.JACS Au. 2024 Jul 26;4(8):2966-2978. doi: 10.1021/jacsau.4c00307. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211606 Free PMC article.
-
Upregulation of Sulfated N-Glycans in Serum as Predictive Biomarkers for Early-Stage Breast Cancer.Int J Mol Sci. 2025 May 22;26(11):4968. doi: 10.3390/ijms26114968. Int J Mol Sci. 2025. PMID: 40507784 Free PMC article.
-
Phosphates as Assisting Groups in Glycan Synthesis.ACS Cent Sci. 2023 Dec 20;10(1):138-142. doi: 10.1021/acscentsci.3c00896. eCollection 2024 Jan 24. ACS Cent Sci. 2023. PMID: 38292611 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
References
-
- Klaassen CD, Boles JW, Sulfation and sulfotransferases 5: the importance of 3’-phosphoadenosine 5’-phosphosulfate (PAPS) in the regulation of sulfation, FASEB J. 11 (6) (1997) 404–418. - PubMed
-
- She Y-M, Li X, Cyr TD, Remarkable Structural Diversity of N-Glycan Sulfation on Influenza Vaccines, Anal. Chem. 91 (8) (2019) 5083–5090. - PubMed
-
- Ichimiya T, Nishihara S, Takase-Yoden S, Kida H, Aoki-Kinoshita K, Frequent glycan structure mining of influenza virus data revealed a sulfated glycan motif that increased viral infection, Bioinformatics 30 (5) (2014) 706–711. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

