Targeting interleukin 4 receptor alpha on tumor-associated macrophages reduces the pro-tumor macrophage phenotype
- PMID: 35939881
- PMCID: PMC9386102
- DOI: 10.1016/j.neo.2022.100830
Targeting interleukin 4 receptor alpha on tumor-associated macrophages reduces the pro-tumor macrophage phenotype
Abstract
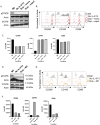
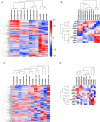
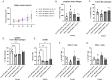
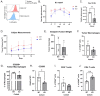
Tumor-associated macrophages (TAMs) are an abundant tumor-promoting cell type in the tumor microenvironment (TME). Most TAMs exhibit a pro-tumor M2-like phenotype supportive of tumor growth, immune evasion, and metastasis. IL-4 and IL-13 are major cytokines that polarize macrophages to an M2 subset and share a common receptor, IL-4 receptor alpha (IL-4R alpha). Treatment of human ex vivo polarized M2 macrophages and M2 macrophage precursors with IL-4R alpha antagonist antibody Dupilumab (DupixentⓇ) reduces M2 macrophage features, including a shift in cell surface marker protein expression and gene expression. In animal models of prostate cancer, both pharmacologic inhibition of IL-4R alpha and genetic deletion of IL-4R alpha utilizing an Il4ra -/- mouse model result in decreased CD206 on TAMs. These data support IL-4R alpha as a target to reduce the pro-tumor, M2-like macrophage phenotype as a novel adjunct cancer therapy.
Keywords: Dupilumab; Dupixent; IL-4; IL-4R alpha; Macrophage; Prostate cancer.
Copyright © 2022. Published by Elsevier Inc.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

