Dysregulation of the kallikrein-kinin system in bronchoalveolar lavage fluid of patients with severe COVID-19
- PMID: 35939907
- PMCID: PMC9352453
- DOI: 10.1016/j.ebiom.2022.104195
Dysregulation of the kallikrein-kinin system in bronchoalveolar lavage fluid of patients with severe COVID-19
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to the angiotensin-converting enzyme 2 (ACE2) receptor, a critical component of the kallikrein-kinin system. Its dysregulation may lead to increased vascular permeability and release of inflammatory chemokines. Interactions between the kallikrein-kinin and the coagulation system might further contribute to thromboembolic complications in COVID-19.
Methods: In this observational study, we measured plasma and tissue kallikrein hydrolytic activity, levels of kinin peptides, and myeloperoxidase (MPO)-DNA complexes as a biomarker for neutrophil extracellular traps (NETs), in bronchoalveolar lavage (BAL) fluid from patients with and without COVID-19.
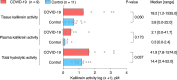
Findings: In BAL fluid from patients with severe COVID-19 (n = 21, of which 19 were mechanically ventilated), we observed higher tissue kallikrein activity (18·2 pM [1·2-1535·0], median [range], n = 9 vs 3·8 [0·0-22·0], n = 11; p = 0·030), higher levels of the kinin peptide bradykinin-(1-5) (89·6 [0·0-2425·0], n = 21 vs 0·0 [0·0-374·0], n = 19, p = 0·001), and higher levels of MPO-DNA complexes (699·0 ng/mL [66·0-142621·0], n = 21 vs 70·5 [9·9-960·0], n = 19, p < 0·001) compared to patients without COVID-19.
Interpretation: Our observations support the hypothesis that dysregulation of the kallikrein-kinin system might occur in mechanically ventilated patients with severe pulmonary disease, which might help to explain the clinical presentation of patients with severe COVID-19 developing pulmonary oedema and thromboembolic complications. Therefore, targeting the kallikrein-kinin system should be further explored as a potential treatment option for patients with severe COVID-19.
Funding: Research Foundation-Flanders (G0G4720N, 1843418N), KU Leuven COVID research fund.
Keywords: Extracellular traps; Kallikreins; Kinins; SARS-CoV-2; Thromboinflammation.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests M.V., B.N., H.C., and J.H.M.F. are employees of Oxurion NV. Oxurion NV and KU Leuven LRD submitted a patent on kallikrein inhibitors. K.M. is an inventor on the granted patent US9642822 awarded to Children's Medical Center Corporation covering the targeting of NETs in thrombosis and lung injury and the pending patent WO20180271953A1. She reports issued patent US9642822B2 and pending patents US2019167680A1. K.M. also reports consulting fees from PEEL Therapeutics. She holds a grant from Flanders Research Foundation (FWO) and a Horizon 2020 grant, as well as a grant from the International Society on Thrombosis and Haemostasis. T.V. is a board member and vice-president of the Belgian Society for Thrombosis and Haemostasis. P.V. and T.V. are co-holders of a research chair for clinical research with Trasylol (DAWN-AntiCo). P.V. holds an institutional grant funded by Bayer AG and by BMS/Pfizer. He reports consulting fees from Anthos Therapeutics, Bayer AG, Boehringer,BMS, Pfizer, Daiichi Sankyo, and Portola/Actelion, and honoraria as a speaker from Bayer, BMS, Pfizer, Daiichi Sankyo, and Leo Pharma. P.V. has participated in a DSMB for clinical trials sponsored by Bayer and Boehringer Ingelheim. R.V. and A.V. report participation in advisory board for Takeda and involvement in ERS, ESOT/ECTTA boards. J.W. is a holder of research grants funded by MSD, Pfizer, and Gilead. He reports speakers fees and support for attending meetings from Gilead, MSD, and Pfizer, and participation on an advisory board for Gilead. He has received study medication for clinical trials from MSD. The other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

