Two Years into the COVID-19 Pandemic: Lessons Learned
- PMID: 35940589
- PMCID: PMC9380879
- DOI: 10.1021/acsinfecdis.2c00204
Two Years into the COVID-19 Pandemic: Lessons Learned
Abstract
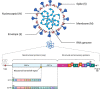
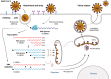
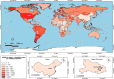
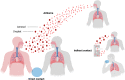
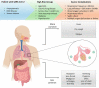
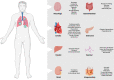
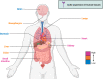
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible and virulent human-infecting coronavirus that emerged in late December 2019 in Wuhan, China, causing a respiratory disease called coronavirus disease 2019 (COVID-19), which has massively impacted global public health and caused widespread disruption to daily life. The crisis caused by COVID-19 has mobilized scientists and public health authorities across the world to rapidly improve our knowledge about this devastating disease, shedding light on its management and control, and spawned the development of new countermeasures. Here we provide an overview of the state of the art of knowledge gained in the last 2 years about the virus and COVID-19, including its origin and natural reservoir hosts, viral etiology, epidemiology, modes of transmission, clinical manifestations, pathophysiology, diagnosis, treatment, prevention, emerging variants, and vaccines, highlighting important differences from previously known highly pathogenic coronaviruses. We also discuss selected key discoveries from each topic and underline the gaps of knowledge for future investigations.
Keywords: SARS-CoV-2; clinical features; diagnosis; pathophysiology; prevention; reservoir hosts; transmission; treatment; vaccines; variants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
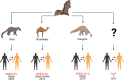



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

