Duck CD8+ T Cell Response to H5N1 Highly Pathogenic Avian Influenza Virus Infection In Vivo and In Vitro
- PMID: 35940633
- PMCID: PMC10613577
- DOI: 10.4049/jimmunol.2101147
Duck CD8+ T Cell Response to H5N1 Highly Pathogenic Avian Influenza Virus Infection In Vivo and In Vitro
Abstract
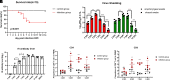
Domestic ducks are the important host for H5N1 highly pathogenic avian influenza virus (HPAIV) infection and epidemiology, but little is known about the duck T cell response to H5N1 AIV infection. In infection experiments of mallard ducks, we detected significantly increased CD8+ cells and augmented expression of cytotoxicity-associated genes, including granzyme A and IFN-γ, in PBMCs from 5 to 9 d postinfection when the virus shedding was clearly decreased, which suggested the importance of the duck cytotoxic T cell response in eliminating H5N1 infection in vivo. Intriguingly, we found that a CD8high+ population of PBMCs was clearly upregulated in infected ducks from 7 to 9 d postinfection compared with uninfected ducks. Next, we used Smart-Seq2 technology to investigate the heterogeneity and transcriptional differences of the duck CD8+ cells. Thus, CD8high+ cells were likely to be more responsive to H5N1 AIV infection, based on the high level of expression of genes involved in T cell responses, activation, and proliferation, including MALT1, ITK, LCK, CD3E, CD247, CFLAR, IL-18R1, and IL-18RAP. More importantly, we have also successfully cultured H5N1 AIV-specific duck T cells in vitro, to our knowledge, for the first time, and demonstrated that the CD8high+ population was increased with the duck T cell activation and response in vitro, which was consistent with results in vivo. Thus, the duck CD8high+ cells represent a potentially effective immune response to H5N1 AIV infection in vivo and in vitro. These findings provide novel insights and direction for developing effective H5N1 AIV vaccines.
Copyright © 2022 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
-
- Ku A. S., Chan L. T.. 1999. The first case of H5N1 avian influenza infection in a human with complications of adult respiratory distress syndrome and Reye’s syndrome. J. Paediatr. Child Health 35: 207–209. - PubMed
-
- Pantin-Jackwood M. J., Suarez D. L.. 2013. Vaccination of domestic ducks against H5N1 HPAI: a review. Virus Res. 178: 21–34. - PubMed
-
- Mishra A., Vijayakumar P., Raut A. A.. 2017. Emerging avian influenza infections: current understanding of innate immune response and molecular pathogenesis. Int. Rev. Immunol. 36: 89–107. - PubMed
-
- Yuk S. S., Lee D. H., Park J. K., To E. O., Kwon J. H., Noh J. Y., Gomis S., Song C. S.. 2015. Immune response in domestic ducks following intradermal delivery of inactivated vaccine against H5N1 highly pathogenic avian influenza virus adjuvanted with oligodeoxynucleotides containing CpG motifs. Poult. Sci. 94: 1836–1842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

