Reporting and transparent research practices in sports medicine and orthopaedic clinical trials: a meta-research study
- PMID: 35940834
- PMCID: PMC9364413
- DOI: 10.1136/bmjopen-2021-059347
Reporting and transparent research practices in sports medicine and orthopaedic clinical trials: a meta-research study
Abstract
Objectives: Transparent reporting of clinical trials is essential to assess the risk of bias and translate research findings into clinical practice. While existing studies have shown that deficiencies are common, detailed empirical and field-specific data are scarce. Therefore, this study aimed to examine current clinical trial reporting and transparent research practices in sports medicine and orthopaedics.
Setting: Exploratory meta-research study on reporting quality and transparent research practices in orthopaedics and sports medicine clinical trials.
Participants: The sample included clinical trials published in the top 25% of sports medicine and orthopaedics journals over 9 months.
Primary and secondary outcome measures: Two independent reviewers assessed pre-registration, open data and criteria related to scientific rigour, like randomisation, blinding, and sample size calculations, as well as the study sample, and data analysis.
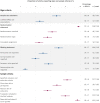
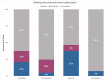
Results: The sample included 163 clinical trials from 27 journals. While the majority of trials mentioned rigour criteria, essential details were often missing. Sixty per cent (95% confidence interval (CI) 53% to 68%) of trials reported sample size calculations, but only 32% (95% CI 25% to 39%) justified the expected effect size. Few trials indicated the blinding status of all main stakeholders (4%; 95% CI 1% to 7%). Only 18% (95% CI 12% to 24%) included information on randomisation type, method and concealed allocation. Most trials reported participants' sex/gender (95%; 95% CI 92% to 98%) and information on inclusion and exclusion criteria (78%; 95% CI 72% to 84%). Only 20% (95% CI 14% to 26%) of trials were pre-registered. No trials deposited data in open repositories.
Conclusions: These results will aid the sports medicine and orthopaedics community in developing tailored interventions to improve reporting. While authors typically mention blinding, randomisation and other factors, essential details are often missing. Greater acceptance of open science practices, like pre-registration and open data, is needed. As these practices have been widely encouraged, we discuss systemic interventions that may improve clinical trial reporting.
Keywords: clinical trials; medical education & training; orthopaedic & trauma surgery; rehabilitation medicine; sports medicine; statistics & research methods.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Up Front and Open? Shrouded in Secrecy? Or Somewhere in Between? A Meta-Research Systematic Review of Open Science Practices in Sport Medicine Research.J Orthop Sports Phys Ther. 2023 Dec;53(12):735-747. doi: 10.2519/jospt.2023.12016. J Orthop Sports Phys Ther. 2023. PMID: 37860866
-
Methodological reporting quality of randomized controlled trials: A survey of seven core journals of orthopaedics from Mainland China over 5 years following the CONSORT statement.Orthop Traumatol Surg Res. 2016 Nov;102(7):933-938. doi: 10.1016/j.otsr.2016.05.018. Epub 2016 Aug 8. Orthop Traumatol Surg Res. 2016. PMID: 27514437
-
Risk of bias of randomized controlled trials published in orthopaedic journals.BMC Med Res Methodol. 2013 Jun 9;13:76. doi: 10.1186/1471-2288-13-76. BMC Med Res Methodol. 2013. PMID: 23758875 Free PMC article.
-
Conservative management following closed reduction of traumatic anterior dislocation of the shoulder.Cochrane Database Syst Rev. 2019 May 10;5(5):CD004962. doi: 10.1002/14651858.CD004962.pub4. Cochrane Database Syst Rev. 2019. PMID: 31074847 Free PMC article.
Cited by
-
A Pilot Study on the Reliability of Ultrasound-Based Assessment of Patella Diameter and Sulcus Angle.Diagnostics (Basel). 2022 Dec 14;12(12):3164. doi: 10.3390/diagnostics12123164. Diagnostics (Basel). 2022. PMID: 36553171 Free PMC article.
-
Is the future of peer review automated?BMC Res Notes. 2022 Jun 11;15(1):203. doi: 10.1186/s13104-022-06080-6. BMC Res Notes. 2022. PMID: 35690782 Free PMC article.
-
GPT for RCTs? Using AI to determine adherence to clinical trial reporting guidelines.BMJ Open. 2025 Mar 18;15(3):e088735. doi: 10.1136/bmjopen-2024-088735. BMJ Open. 2025. PMID: 40107689 Free PMC article.
-
Checklists, risk of bias tools, and reporting guidelines for research in orthopedics, sports medicine, and rehabilitation.Knee Surg Sports Traumatol Arthrosc. 2023 Aug;31(8):3029-3033. doi: 10.1007/s00167-023-07442-8. Epub 2023 May 5. Knee Surg Sports Traumatol Arthrosc. 2023. PMID: 37145131 Review. No abstract available.
-
Assessing transparency practices in dental randomized controlled trials.BMC Med Res Methodol. 2024 Aug 24;24(1):185. doi: 10.1186/s12874-024-02316-0. BMC Med Res Methodol. 2024. PMID: 39182028 Free PMC article.
References
-
- Califf RM, DeMets DL. Principles from clinical trials relevant to clinical practice: Part I. Circulation 2002;106:1015–21. 10.1161/01.cir.0000023260.78078.bb - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
