Pre S cription Digita L Th E rap E utic for P atients with I nsomnia (SLEEP-I): a protocol for a pragmatic randomised controlled trial
- PMID: 35940841
- PMCID: PMC9364397
- DOI: 10.1136/bmjopen-2022-062041
Pre S cription Digita L Th E rap E utic for P atients with I nsomnia (SLEEP-I): a protocol for a pragmatic randomised controlled trial
Abstract
Introduction: Cognitive behavioural therapy for insomnia (CBT-I) is effective at treating chronic insomnia, yet in-person CBT-I can often be challenging to access. Prior studies have used technology to bridge barriers but have been unable to extensively assess the impact of the digital therapeutic on real-world patient experience and multidimensional outcomes. Among patients with insomnia, our aim is to determine the impact of a prescription digital therapeutic (PDT) (PEAR-003b, FDA-authorised as Somryst; herein called PDT) that provides mobile-delivered CBT-I on patient-reported outcomes (PROs) and healthcare utilisation.
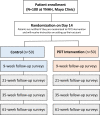
Methods and analysis: We are conducting a pragmatically designed, prospective, multicentre randomised controlled trial that leverages Hugo, a unique patient-centred health data-aggregating platform for data collection and patient follow-up from Hugo Health. A total of 100 participants with insomnia from two health centres will be enrolled onto the Hugo Health platform, provided with a linked Fitbit (Inspire 2) to track activity and then randomised 1:1 to receive (or not) the PDT for mobile-delivered CBT-I (Somryst). The primary outcome is a change in the insomnia severity index score from baseline to 9-week postrandomisation. Secondary outcomes include healthcare utilisation, health utility scores and clinical outcomes; change in sleep outcomes as measured with sleep diaries and a change in individual PROs including depressive symptoms, daytime sleepiness, health status, stress and anxiety. For those allocated to the PDT, we will also assess engagement with the PDT.
Ethics and dissemination: The Institutional Review Boards at Yale University and the Mayo Clinic have approved the trial protocol. This trial will provide important data to patients, clinicians and policymakers about the impact of the PDT device delivering CBT-I on PROs, clinical outcomes and healthcare utilisation. Findings will be disseminated to participants, presented at professional meetings and published in peer-reviewed journals.
Trial registration number: NCT04909229.
Keywords: Clinical trials; MENTAL HEALTH; SLEEP MEDICINE.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RPD reports research funding from the American Heart Association, the Canadian Institutes of Health Research and from the Medical Device Innovation Consortium (MDIC) as part of the National Evaluation System for health Technology Coordinating Center (NESTcc), Food and Drug Administration (FDA). JSR received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the FDA to establish the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), MDIC as part of the NESTcc, AHRQ (R01HS022882), NHLBI of the NIH (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale. NDS is currently employed by Delta Airlines; when he was employed by the Mayo Clinic, he reported receiving research support through Mayo Clinic from the Food and Drug Administration, the Centers of Medicare & Medicaid Innovation under the Transforming Clinical Practice Initiative, the Agency for Healthcare Research and Quality (grants R01HS025164, R01HS025402, R03HS025517 and K12HS026379), the National Heart, Lung and Blood Institute of the National Institutes of Health (grants R56HL130496, R01HL131535 and R01HL151662), the National Science Foundation, the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology, and the Patient-Centered Outcomes Research Institute to develop a Clinical Data Research Network. FT is an employee of Pear Therapeutics, that develops and distributes prescription digital therapeutics for health concerns and disease. Other authors report no other conflicts of interest.
Figures
References
-
- Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic Criteria/International classification of sleep disorders, second edition criteria: results from the America insomnia survey. Biol Psychiatry 2011;69:592–600. 10.1016/j.biopsych.2010.10.023 - DOI - PubMed
-
- Fullerton DSP. The economic impact of insomnia in managed care: a clearer picture emerges. Am J Manag Care 2006;12:S246–52. - PubMed
-
- Stoller MK. Economic effects of insomnia. Clin Ther 1994;16:873–97. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous