Association of Microglial Activation With Spontaneous ARIA-E and CSF Levels of Anti-Aβ Autoantibodies
- PMID: 35940900
- PMCID: PMC9576297
- DOI: 10.1212/WNL.0000000000200892
Association of Microglial Activation With Spontaneous ARIA-E and CSF Levels of Anti-Aβ Autoantibodies
Abstract
Background and objectives: Amyloid-related imaging abnormalities suggestive of vasogenic edema or sulcal effusion (ARIA-E) are the most common adverse events complicating Alzheimer disease (AD) immunotherapy with anti-β-amyloid (Aβ) monoclonal antibodies. ARIA-E can also occur spontaneously in cerebral amyloid angiopathy-related inflammation (CAA-ri), a rare autoimmune encephalopathy associated with increased CSF levels of anti-Aβ autoantibodies. Although the pathophysiologic mechanisms of ARIA-E remain to be fully elucidated, experimental evidence from ex vivo studies suggests that gantenerumab and aducanumab enable microglial activation. However, the in vivo evidence for a direct association between neuroinflammation and ARIA-E in patients with high CSF anti-Aβ (auto)antibody levels has never been demonstrated.
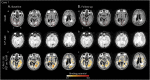
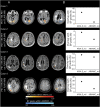
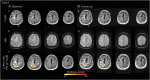
Methods: The spatial distribution and temporal variations of microglial activation associated with levels of anti-Aβ autoantibodies at (sub)acute presentation of ARIA-E and after corticosteroid therapy were evaluated in a longitudinal case series of patients with CAA-ri, the spontaneous variant of the iatrogenic ARIA-E reported in Aβ-lowering immunotherapy with monoclonal antibodies. Multimodal and multiparametric MRI was used for CAA and ARIA-E severity quantification, according to validated scoring system; CSF testing for anti-Aβ autoantibodies and AD biomarkers; 11C-PK11195 PET for activated microglia.
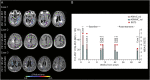
Results: At (sub)acute presentation, we found focal peaks of microglial activation having a greater spatial colocalization with ARIA-E compared with chronic age-related white matter change imaging abnormalities. The severity of ARIA-E and the magnitude of the associated microglial activation were greater in patients having AD and severe CAA concomitant disease compared with patients having CAA only. CSF anti-Aβ autoantibodies at presentation were high in all patients and markedly decreased at posttreatment follow-up, in parallel with clinical resolution of acute symptoms, reduced ARIA-E severity, and reduced microglial activation.
Discussion: Our findings extend the current notion of ARIA-E by providing the first in vivo 11C-PK11195 PET evidence for an association between microglial activation and the magnitude and severity of ARIA-E in patients with increased CSF concentration of anti-Aβ autoantibodies and comorbid AD and CAA disease. Our results highlight CSF testing for anti-Aβ autoantibodies as a promising diagnostic, prognostic, and therapy response biomarker to help guide future treatment and management decisions in real clinical practice and clinical trials.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Piazza F, Greenberg SM, Savoiardo M, et al. Anti-amyloid beta autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol. 2013;73(4):449-458. - PubMed
-
- Barakos J, Purcell D, Suhy J, et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with anti-amyloid beta therapy. J Prev Alz Dis. 2022;9(2):211-220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
