Sorting and Export of Proteins at the Endoplasmic Reticulum
- PMID: 35940902
- PMCID: PMC10153803
- DOI: 10.1101/cshperspect.a041258
Sorting and Export of Proteins at the Endoplasmic Reticulum
Abstract
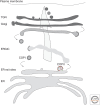
Secretory proteins are transported from the endoplasmic reticulum (ER) to the Golgi complex in carriers that are formed by the concerted activities of cytoplasmic proteins in the coat protein complex II (COPII). COPII was first described in Saccharomyces cerevisiae and its basic functions are largely conserved throughout eukaryotes. The discovery of the TANGO1 (transport and Golgi organization 1) family of proteins is revealing insights into how cells can adapt COPII proteins to reorganize the ER exit site for the export of the most abundant and bulky molecules, collagens.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


Similar articles
-
COPII and the regulation of protein sorting in mammals.Nat Cell Biol. 2011 Dec 22;14(1):20-8. doi: 10.1038/ncb2390. Nat Cell Biol. 2011. PMID: 22193160 Review.
-
Protein export at the ER: loading big collagens into COPII carriers.EMBO J. 2011 Aug 31;30(17):3475-80. doi: 10.1038/emboj.2011.255. EMBO J. 2011. PMID: 21878990 Free PMC article. Review.
-
Vesicle-mediated export from the ER: COPII coat function and regulation.Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review.
-
Tango1 coordinates the formation of endoplasmic reticulum/Golgi docking sites to mediate secretory granule formation.J Biol Chem. 2019 Dec 20;294(51):19498-19510. doi: 10.1074/jbc.RA119.011063. Epub 2019 Nov 5. J Biol Chem. 2019. PMID: 31690624 Free PMC article.
-
TANGO1 assembles into rings around COPII coats at ER exit sites.J Cell Biol. 2017 Apr 3;216(4):901-909. doi: 10.1083/jcb.201608080. Epub 2017 Mar 9. J Cell Biol. 2017. PMID: 28280121 Free PMC article.
Cited by
-
Adaptations of membrane trafficking in cancer and tumorigenesis.J Cell Sci. 2024 May 15;137(10):jcs260943. doi: 10.1242/jcs.260943. Epub 2024 May 21. J Cell Sci. 2024. PMID: 38770683 Free PMC article. Review.
-
TFG regulates inner COPII coat recruitment to facilitate anterograde secretory protein transport.Mol Biol Cell. 2024 Aug 1;35(8):ar113. doi: 10.1091/mbc.E24-06-0282. Epub 2024 Jul 10. Mol Biol Cell. 2024. PMID: 38985515 Free PMC article.
-
Editorial: Homeostatic regulation of protein synthesis, folding and secretion by stress response pathways in eukaryotes.Front Cell Dev Biol. 2023 Sep 13;11:1282272. doi: 10.3389/fcell.2023.1282272. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37779897 Free PMC article. No abstract available.
-
The secretory pathway in Tetrahymena is organized for efficient constitutive secretion at ciliary pockets.iScience. 2024 Oct 9;27(11):111123. doi: 10.1016/j.isci.2024.111123. eCollection 2024 Nov 15. iScience. 2024. PMID: 39498308 Free PMC article.
-
Targets and Potential Mechanism of Chondroitin Sulfate A-selenium Nanoparticle on Kashin-Beck Disease Chondrocytes.Biol Trace Elem Res. 2025 Mar 26. doi: 10.1007/s12011-025-04584-3. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40138104
References
-
- Amodio G, Renna M, Paladino S, Venturi C, Tacchetti C, Moltedo O, Franceschelli S, Mallardo M, Bonatti S, Remondelli P. 2009. Endoplasmic reticulum stress reduces the export from the ER and alters the architecture of post-ER compartments. Int J Biochem Cell Biol 41: 2511–2521. 10.1016/j.biocel.2009.08.006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
