ER-Phagy: Quality and Quantity Control of the Endoplasmic Reticulum by Autophagy
- PMID: 35940904
- PMCID: PMC9808580
- DOI: 10.1101/cshperspect.a041256
ER-Phagy: Quality and Quantity Control of the Endoplasmic Reticulum by Autophagy
Abstract
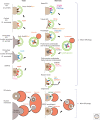
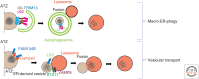
The endoplasmic reticulum (ER) is the largest organelle and has multiple roles in various cellular processes such as protein secretion, lipid synthesis, calcium storage, and organelle biogenesis. The quantity and quality of this organelle are controlled by the ubiquitin-proteasome system and autophagy (termed "ER-phagy"). ER-phagy is defined as the degradation of part of the ER by the vacuole or lysosomes, and there are at least two types of ER-phagy: macro-ER-phagy and micro-ER-phagy. In macro-ER-phagy, ER fragments are enclosed by autophagosomes, which is mediated by ER-phagy receptors. In micro-ER-phagy, a portion of the ER is engulfed directly by the vacuole or lysosomes. In these two pathways, some proteins in the ER lumen can be recognized selectively and subjected to ER-phagy. This review summarizes our current knowledge of ER-phagy, focusing on its membrane dynamics, molecular mechanisms, substrate specificity, and physiological significance.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. 2008. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701. 10.1083/jcb.200803137 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
