Regulation and Functions of the ER-Associated Nrf1 Transcription Factor
- PMID: 35940907
- PMCID: PMC9808582
- DOI: 10.1101/cshperspect.a041266
Regulation and Functions of the ER-Associated Nrf1 Transcription Factor
Abstract
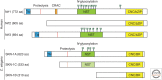
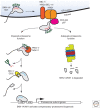
Nrf1 is a member of the nuclear erythroid 2-like family of transcription factors that regulate stress-responsive gene expression in animals. Newly synthesized Nrf1 is targeted to the endoplasmic reticulum (ER) where it is N-glycosylated. N-glycosylated Nrf1 is trafficked to the cytosol by the ER-associated degradation (ERAD) machinery and is subject to rapid proteasomal degradation. When proteasome function is impaired, Nrf1 escapes degradation and undergoes proteolytic cleavage and deglycosylation. Deglycosylation results in deamidation of N-glycosylated asparagine residues to edit the protein sequence encoded by the genome. This truncated and "sequence-edited" form of Nrf1 enters the nucleus where it induces up-regulation of proteasome subunit genes. Thus, Nrf1 drives compensatory proteasome biogenesis in cells exposed to proteasome inhibitor drugs and other proteotoxic insults. In addition to its role in proteasome homeostasis, Nrf1 is implicated in responses to oxidative stress, and maintaining lipid and cholesterol homeostasis. Here, we describe the conserved and complex mechanism by which Nrf1 is regulated and highlight emerging evidence linking this unusual transcription factor to development, aging, and disease.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Protein Sequence Editing of SKN-1A/Nrf1 by Peptide:N-Glycanase Controls Proteasome Gene Expression.Cell. 2019 Apr 18;177(3):737-750.e15. doi: 10.1016/j.cell.2019.03.035. Cell. 2019. PMID: 31002798 Free PMC article.
-
Disabling the Protease DDI2 Attenuates the Transcriptional Activity of NRF1 and Potentiates Proteasome Inhibitor Cytotoxicity.Int J Mol Sci. 2020 Jan 3;21(1):327. doi: 10.3390/ijms21010327. Int J Mol Sci. 2020. PMID: 31947743 Free PMC article.
-
Regulation of NRF1, a master transcription factor of proteasome genes: implications for cancer and neurodegeneration.Mol Biol Cell. 2020 Sep 15;31(20):2158-2163. doi: 10.1091/mbc.E20-04-0238. Mol Biol Cell. 2020. PMID: 32924844 Free PMC article.
-
ER-Resident Transcription Factor Nrf1 Regulates Proteasome Expression and Beyond.Int J Mol Sci. 2020 May 23;21(10):3683. doi: 10.3390/ijms21103683. Int J Mol Sci. 2020. PMID: 32456207 Free PMC article. Review.
-
Changing gears in Nrf1 research, from mechanisms of regulation to its role in disease and prevention.Biochim Biophys Acta. 2015 Oct;1849(10):1260-76. doi: 10.1016/j.bbagrm.2015.08.001. Epub 2015 Aug 5. Biochim Biophys Acta. 2015. PMID: 26254094 Review.
Cited by
-
Predictive modeling of moonlighting DNA-binding proteins.NAR Genom Bioinform. 2022 Dec 2;4(4):lqac091. doi: 10.1093/nargab/lqac091. eCollection 2022 Dec. NAR Genom Bioinform. 2022. PMID: 36474806 Free PMC article.
-
Temporal control of acute protein aggregate turnover by UBE3C and NRF1-dependent proteasomal pathways.Proc Natl Acad Sci U S A. 2024 Dec 10;121(50):e2417390121. doi: 10.1073/pnas.2417390121. Epub 2024 Dec 5. Proc Natl Acad Sci U S A. 2024. PMID: 39636856 Free PMC article.
-
The transcription factor NRF1 (NFE2L1) activates aggrephagy by inducing p62 and GABARAPL1 after proteasome inhibition to maintain proteostasis.Sci Rep. 2023 Sep 1;13(1):14405. doi: 10.1038/s41598-023-41492-9. Sci Rep. 2023. PMID: 37658135 Free PMC article.
-
Disease related changes in ATAC-seq of iPSC-derived motor neuron lines from ALS patients and controls.Nat Commun. 2024 May 2;15(1):3606. doi: 10.1038/s41467-024-47758-8. Nat Commun. 2024. PMID: 38697975 Free PMC article.
-
Transcription factor Nrf1 regulates proteotoxic stress-induced autophagy.J Cell Biol. 2024 Jun 3;223(6):e202306150. doi: 10.1083/jcb.202306150. Epub 2024 Apr 24. J Cell Biol. 2024. PMID: 38656405 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
