Cholesterol Transport to the Endoplasmic Reticulum
- PMID: 35940908
- PMCID: PMC9899650
- DOI: 10.1101/cshperspect.a041263
Cholesterol Transport to the Endoplasmic Reticulum
Abstract
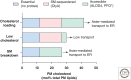
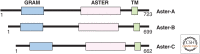
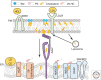
Most cholesterol in mammalian cells is stored in the plasma membrane (PM). Cholesterol transport from the PM to low-sterol regulatory regions of the endoplasmic reticulum (ER) controls cholesterol synthesis and uptake, and thereby influences the rates of cholesterol flux between tissues of complex organisms. Cholesterol transfer to the ER is also required for steroidogenesis, oxysterol and bile acid synthesis, and cholesterol esterification. The ER-resident Aster proteins (Aster-A, -B, and -C) form contacts with the PM to move cholesterol to the ER in mammals. Mice lacking Aster-B have low adrenal cholesteryl ester stores and impaired steroidogenesis because of a defect in cholesterol transport from high-density lipoprotein (HDL) to the ER. This work reviews the molecular characteristics of Asters, their role in HDL- and low-density lipoprotein (LDL)-cholesterol movement, and how cholesterol transferred to the ER is utilized by cells. The roles of other lipid transporters and of membrane lipid organization in maintaining aspects of cholesterol homeostasis are also highlighted.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Interplay between Asters/GRAMD1s and phosphatidylserine in intermembrane transport of LDL cholesterol.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2120411119. doi: 10.1073/pnas.2120411119. Proc Natl Acad Sci U S A. 2022. PMID: 34992143 Free PMC article.
-
Aster Proteins Facilitate Nonvesicular Plasma Membrane to ER Cholesterol Transport in Mammalian Cells.Cell. 2018 Oct 4;175(2):514-529.e20. doi: 10.1016/j.cell.2018.08.033. Epub 2018 Sep 13. Cell. 2018. PMID: 30220461 Free PMC article.
-
Selective Aster inhibitors distinguish vesicular and nonvesicular sterol transport mechanisms.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2024149118. doi: 10.1073/pnas.2024149118. Proc Natl Acad Sci U S A. 2021. PMID: 33376205 Free PMC article.
-
GRAMD1-mediated accessible cholesterol sensing and transport.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Aug;1866(8):158957. doi: 10.1016/j.bbalip.2021.158957. Epub 2021 Apr 28. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33932585 Review.
-
Intracellular cholesterol transport.Arterioscler Thromb Vasc Biol. 2004 Jul;24(7):1150-60. doi: 10.1161/01.ATV.0000131264.66417.d5. Epub 2004 May 6. Arterioscler Thromb Vasc Biol. 2004. PMID: 15130918 Review.
Cited by
-
Intracellular Cholesterol Trafficking.Cold Spring Harb Perspect Biol. 2023 Aug 1;15(8):a041404. doi: 10.1101/cshperspect.a041404. Cold Spring Harb Perspect Biol. 2023. PMID: 37277190 Free PMC article. Review.
-
Hepatic nonvesicular cholesterol transport is critical for systemic lipid homeostasis.Nat Metab. 2023 Jan;5(1):165-181. doi: 10.1038/s42255-022-00722-6. Epub 2023 Jan 16. Nat Metab. 2023. PMID: 36646756 Free PMC article.
-
Toxoplasma gondii sustains survival by regulating cholesterol biosynthesis and uptake via SREBP2 activation.J Lipid Res. 2024 Dec;65(12):100684. doi: 10.1016/j.jlr.2024.100684. Epub 2024 Oct 28. J Lipid Res. 2024. PMID: 39490926 Free PMC article.
-
Aster-B-dependent estradiol synthesis protects female mice from diet-induced obesity.J Clin Invest. 2024 Jan 4;134(4):e173002. doi: 10.1172/JCI173002. J Clin Invest. 2024. PMID: 38175723 Free PMC article.
-
Regulation of cellular cholesterol distribution via non-vesicular lipid transport at ER-Golgi contact sites.Nat Commun. 2023 Sep 21;14(1):5867. doi: 10.1038/s41467-023-41213-w. Nat Commun. 2023. PMID: 37735529 Free PMC article.
References
-
- Abrams ME, Johnson KA, Perelman SS, Zhang LS, Endapally S, Mar KB, Thompson BM, McDonald JG, Schoggins JW, Radhakrishnan A, et al. 2020. Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol. Nat Microbiol 5: 929–942. 10.1038/s41564-020-0701-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
