Endoplasmic Reticulum Architecture and Inter-Organelle Communication in Metabolic Health and Disease
- PMID: 35940911
- PMCID: PMC9899651
- DOI: 10.1101/cshperspect.a041261
Endoplasmic Reticulum Architecture and Inter-Organelle Communication in Metabolic Health and Disease
Abstract
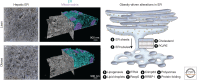
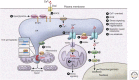
The endoplasmic reticulum (ER) is a key organelle involved in the regulation of lipid and glucose metabolism, proteostasis, Ca2+ signaling, and detoxification. The structural organization of the ER is very dynamic and complex, with distinct subdomains such as the nuclear envelope and the peripheral ER organized into ER sheets and tubules. ER also forms physical contact sites with all other cellular organelles and with the plasma membrane. Both form and function of the ER are highly adaptive, with a potent capacity to respond to transient changes in environmental cues such as nutritional fluctuations. However, under obesity-induced chronic stress, the ER fails to adapt, leading to ER dysfunction and the development of metabolic pathologies such as insulin resistance and fatty liver disease. Here, we discuss how the remodeling of ER structure and contact sites with other organelles results in diversification of metabolic function and how perturbations to this structural flexibility by chronic overnutrition contribute to ER dysfunction and metabolic pathologies in obesity.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
