Making more COVID-19 vaccines available to address global needs: Considerations and a framework for their evaluation
- PMID: 35941036
- PMCID: PMC9343170
- DOI: 10.1016/j.vaccine.2022.07.028
Making more COVID-19 vaccines available to address global needs: Considerations and a framework for their evaluation
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
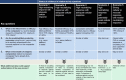
Figures
References
-
- WHO Target Product Profiles for COVID-19 Vaccines, Revised version, April 2022. Geneva: World Health Organization; 2022. <https://cdn.who.int/media/docs/default-source/blue-print/tpp-6apr-2022-f... [retrieved 31 May 2022].
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. - PubMed
-
- Developing a framework for evaluating new COVID-19 vaccines. Geneva: World Health Organization; 2022. <https://www.who.int/news-room/events/detail/2022/02/23/default-calendar/... [retrieved 31 May 2022].
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

