Acetate reprograms gut microbiota during alcohol consumption
- PMID: 35941112
- PMCID: PMC9359997
- DOI: 10.1038/s41467-022-31973-2
Acetate reprograms gut microbiota during alcohol consumption
Abstract
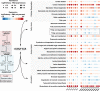
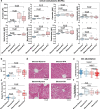
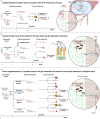
Liver damage due to chronic alcohol use is among the most prevalent liver diseases. Alcohol consumption frequency is a strong factor of microbiota variance. Here we use isotope labeled [1-13C] ethanol, metagenomics, and metatranscriptomics in ethanol-feeding and intragastric mouse models to investigate the metabolic impacts of alcohol consumption on the gut microbiota. First, we show that although stable isotope labeled [1-13C] ethanol contributes to fatty acid pools in the liver, plasma, and cecum contents of mice, there is no evidence of ethanol metabolism by gut microbiota ex vivo under anaerobic conditions. Next, we observe through metatranscriptomics that the gut microbiota responds to ethanol-feeding by activating acetate dissimilation, not by metabolizing ethanol directly. We demonstrate that blood acetate concentrations are elevated during ethanol consumption. Finally, by increasing systemic acetate levels with glyceryl triacetate supplementation, we do not observe any impact on liver disease, but do induce similar gut microbiota alterations as chronic ethanol-feeding in mice. Our results show that ethanol is not directly metabolized by the gut microbiota, and changes in the gut microbiota linked to ethanol are a side effect of elevated acetate levels. De-trending for these acetate effects may be critical for understanding gut microbiota changes that cause alcohol-related liver disease.
© 2022. The Author(s).
Conflict of interest statement
B.S. has been consulting for Ferring Research Institute, Gelesis, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals and Takeda. B.S.’s institution UC San Diego has received research support from Axial Biotherapeutics, BiomX, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. B.S. is founder of Nterica Bio. UC San Diego has filed several patents with B.S. as an inventor related to this work. The remaining authors declare no competing interests.
Figures