Nrf2 signaling activation by a small molecule activator compound 16 inhibits hydrogen peroxide-induced oxidative injury and death in osteoblasts
- PMID: 35941127
- PMCID: PMC9360014
- DOI: 10.1038/s41420-022-01146-7
Nrf2 signaling activation by a small molecule activator compound 16 inhibits hydrogen peroxide-induced oxidative injury and death in osteoblasts
Abstract
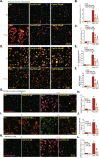
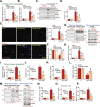
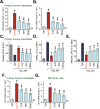
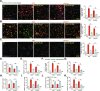
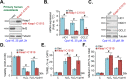
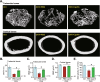
We explored the potential activity of compound 16 (Cpd16), a novel small molecule Nrf2 activator, in hydrogen peroxide (H2O2)-stimulated osteoblasts. In the primary murine/human osteoblasts and MC3T3-E1 murine osteoblastic cells, Cpd16 treatment at micro-molar concentrations caused disassociation of Keap1-Nrf2 and Nrf2 cascade activation. Cpd16 induced stabilization of Nrf2 protein and its nuclear translocation, thereby increasing the antioxidant response elements (ARE) reporter activity and Nrf2 response genes transcription in murine and human osteoblasts. Significantly, Cpd16 mitigated oxidative injury in H2O2-stimulited osteoblasts. H2O2-provoked apoptosis as well as programmed necrosis in osteoblasts were significantly alleviated by the novel Nrf2 activator. Cpd16-induced Nrf2 activation and osteoblasts protection were stronger than other known Nrf2 activators. Dexamethasone- and nicotine-caused oxidative stress and death in osteoblasts were attenuated by Cpd16 as well. Cpd16-induced osteoblast cytoprotection was abolished by Nrf2 short hairpin RNA or knockout, but was mimicked by Keap1 knockout. Keap1 Cys151S mutation abolished Cpd16-induced Nrf2 cascade activation and osteoblasts protection against H2O2. Importantly, weekly Cpd16 administration largely ameliorated trabecular bone loss in ovariectomy mice. Together, Cpd16 alleviates H2O2-induced oxidative stress and death in osteoblasts by activating Nrf2 cascade.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A novel Keap1 inhibitor iKeap1 activates Nrf2 signaling and ameliorates hydrogen peroxide-induced oxidative injury and apoptosis in osteoblasts.Cell Death Dis. 2021 Jul 5;12(7):679. doi: 10.1038/s41419-021-03962-8. Cell Death Dis. 2021. PMID: 34226516 Free PMC article.
-
Neuroligin-3 activates Akt-dependent Nrf2 cascade to protect osteoblasts from oxidative stress.Free Radic Biol Med. 2023 Nov 1;208:807-819. doi: 10.1016/j.freeradbiomed.2023.09.032. Epub 2023 Sep 27. Free Radic Biol Med. 2023. PMID: 37774803
-
Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury.Cell Death Dis. 2020 Sep 17;11(9):772. doi: 10.1038/s41419-020-02987-9. Cell Death Dis. 2020. PMID: 32943614 Free PMC article.
-
Activation of Nrf2 by MIND4-17 protects osteoblasts from hydrogen peroxide-induced oxidative stress.Oncotarget. 2017 Nov 10;8(62):105662-105672. doi: 10.18632/oncotarget.22360. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285281 Free PMC article.
-
Canonical and non-canonical mechanisms of Nrf2 activation.Pharmacol Res. 2018 Aug;134:92-99. doi: 10.1016/j.phrs.2018.06.013. Epub 2018 Jun 18. Pharmacol Res. 2018. PMID: 29913224 Review.
Cited by
-
Research progress of autoimmune diseases based on induced pluripotent stem cells.Front Immunol. 2024 Apr 24;15:1349138. doi: 10.3389/fimmu.2024.1349138. eCollection 2024. Front Immunol. 2024. PMID: 38720903 Free PMC article. Review.
-
MiR-144-5p and miR-21-5p do not drive bone disease in a mouse model of type 1 diabetes mellitus.JBMR Plus. 2024 Apr 6;8(5):ziae036. doi: 10.1093/jbmrpl/ziae036. eCollection 2024 May. JBMR Plus. 2024. PMID: 38606150 Free PMC article.
-
Emerging Therapeutic Targets in Rheumatoid Arthritis: Focusing on HIF-1α, Nrf2, STATs, and RORγt.Curr Drug Targets. 2025;26(8):507-533. doi: 10.2174/0113894501372670250408074908. Curr Drug Targets. 2025. PMID: 40247798 Review.
References
LinkOut - more resources
Full Text Sources

