Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose
- PMID: 35941158
- PMCID: PMC9358634
- DOI: 10.1038/s41467-022-32312-1
Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose
Abstract
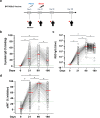
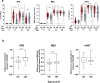
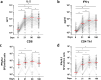
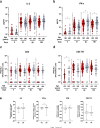
Understanding the impact of age on vaccinations is essential for the design and delivery of vaccines against SARS-CoV-2. Here, we present findings from a comprehensive analysis of multiple compartments of the memory immune response in 312 individuals vaccinated with the BNT162b2 SARS-CoV-2 mRNA vaccine. Two vaccine doses induce high antibody and T cell responses in most individuals. However, antibody recognition of the Spike protein of the Delta and Omicron variants is less efficient than that of the ancestral Wuhan strain. Age-stratified analyses identify a group of low antibody responders where individuals ≥60 years are overrepresented. Waning of the antibody and cellular responses is observed in 30% of the vaccinees after 6 months. However, age does not influence the waning of these responses. Taken together, while individuals ≥60 years old take longer to acquire vaccine-induced immunity, they develop more sustained acquired immunity at 6 months post-vaccination. A third dose strongly boosts the low antibody responses in the older individuals against the ancestral Wuhan strain, Delta and Omicron variants.
© 2022. The Author(s).
Conflict of interest statement
A patent application for the SFB assay has been filed (Singapore patent #10202009679 P: A Method Of Detecting Antibodies And Related Products) (L.R., Y.S.G., and L.F.P.N). The authors declare no other competing interests.
Figures



References
-
- Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

