Nitrogen cycling and microbial cooperation in the terrestrial subsurface
- PMID: 35941171
- PMCID: PMC9562985
- DOI: 10.1038/s41396-022-01300-0
Nitrogen cycling and microbial cooperation in the terrestrial subsurface
Abstract
The nitrogen cycle plays a major role in aquatic nitrogen transformations, including in the terrestrial subsurface. However, the variety of transformations remains understudied. To determine how nitrogen cycling microorganisms respond to different aquifer chemistries, we sampled groundwater with varying nutrient and oxygen contents. Genes and transcripts involved in major nitrogen-cycling pathways were quantified from 55 and 26 sites, respectively, and metagenomes and metatranscriptomes were analyzed from a subset of oxic and dysoxic sites (0.3-1.1 mg/L bulk dissolved oxygen). Nitrogen-cycling mechanisms (e.g. ammonia oxidation, denitrification, dissimilatory nitrate reduction to ammonium) were prevalent and highly redundant, regardless of site-specific physicochemistry or nitrate availability, and present in 40% of reconstructed genomes, suggesting that nitrogen cycling is a core function of aquifer communities. Transcriptional activity for nitrification, denitrification, nitrite-dependent anaerobic methane oxidation and anaerobic ammonia oxidation (anammox) occurred simultaneously in oxic and dysoxic groundwater, indicating the availability of oxic-anoxic interfaces. Concurrent activity by these microorganisms indicates potential synergisms through metabolite exchange across these interfaces (e.g. nitrite and oxygen). Fragmented denitrification pathway encoding and transcription was widespread among groundwater bacteria, although a considerable proportion of associated transcriptional activity was driven by complete denitrifiers, especially under dysoxic conditions. Despite large differences in transcription, the capacity for the final steps of denitrification was largely invariant to aquifer conditions, and most genes and transcripts encoding N2O reductases were the atypical Sec-dependant type, suggesting energy-efficiency prioritization. Results provide insights into the capacity for cooperative relationships in groundwater communities, and the richness and complexity of metabolic mechanisms leading to the loss of fixed nitrogen.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



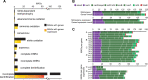

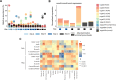

References
-
- Taylor RG, Scanlon B, Döll P, Rodell M, Van Beek R, Wada Y, et al. Groundwater and climate change. Nat Clim Chang. 2013;3:322–9. doi: 10.1038/nclimate1744. - DOI
-
- Aeschbach-Hertig W, Gleeson T. Regional strategies for the accelerating global problem of groundwater depletion. Nat Geosci. 2012;5:853–61. doi: 10.1038/ngeo1617. - DOI
-
- Morgenstern U, Daughney CJ. Groundwater age for identification of baseline groundwater quality and impacts of land-use intensification - The National Groundwater Monitoring Programme of New Zealand. J Hydrol. 2012;456–457:79–93. doi: 10.1016/j.jhydrol.2012.06.010. - DOI

