Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial
- PMID: 35941372
- PMCID: PMC9499862
- DOI: 10.1038/s41591-022-01935-8
Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial
Abstract
Trastuzumab deruxtecan is an antibody-drug conjugate with high extracranial activity in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. We conducted the prospective, open-label, single-arm, phase 2 TUXEDO-1 trial. We enrolled patients aged ≥18 years with HER2-positive breast cancer and newly diagnosed untreated brain metastases or brain metastases progressing after previous local therapy, previous exposure to trastuzumab and pertuzumab and no indication for immediate local therapy. Patients received trastuzumab deruxtecan intravenously at the standard dose of 5.4 mg per kg bodyweight once every 3 weeks. The primary endpoint was intracranial response rate measured according to the response assessment in neuro-oncology brain metastases criteria. A Simon two-stage design was used to compare a null hypothesis of <26% response rate against an alternative of 61%. Fifteen patients were enrolled in the intention-to-treat population of patients who received at least one dose of study drug. Two patients (13.3%) had a complete intracranial response, nine (60%) had a partial intracranial response and three (20%) had stable disease as the best intracranial response, with a best overall intracranial response rate of 73.3% (95% confidential interval 48.1-89.1%), thus meeting the predefined primary outcome. No new safety signals were observed and global quality-of-life and cognitive functioning were maintained over the treatment duration. In the TUXEDO-1 trial (NCT04752059, EudraCT 2020-000981-41), trastuzumab deruxtecan showed a high intracranial response rate in patients with active brain metastases from HER2-positive breast cancer and should be considered as a treatment option in this setting.
© 2022. The Author(s).
Conflict of interest statement
R.B. has received honoraria for advisory role from Astra Zeneca, Daiichi, Eisai, Eli Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche, Seagen, lecture honoraria from Astra Zeneca, Celgene, Eli Lilly, Novartis, Pfizer, Pierre-Fabre, Roche, Seagen and research support from Daiichi, MSD, Novartis and Roche. A.S.B. has research support from Daiichi Sankyo and Roche, honoraria for lectures, consultation or advisory board participation for Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo as well as travel support from Roche, Amgen, Daiichi Sankyo and AbbVie. A.M.S. has received honoraria for lectures from Astra Zeneca and travel support from PharmaMar. Z.B.H. has received honoraria for advisory board from MSD, Roche, congress funding from Daichii Sankyo and lecture honoraria from MSD and Daichii Sankyo. H.H. reports grants from Glock Health Science and Research, BlueSky Immunotherapies and the Austrian Ministry of Education Science and Research. A.I.M. reports honoraria for participation in advisory boards organized by MSD, Servier and BMS, lecture honoraria from Eli Lilly, Servier, BMS and MSD, consulting for Astellas and MSD and travel support from BMS, Roche, Eli Lilly and Daiichi Sankyo. C.M. received honoraria from Boehringer Ingelheim, MSD, Amgen and travel grants from MSD and Merck Darmstadt. T.F. reports honoraria or being an advisor for MSD, Merck, BMS, Böhringer Ingelheim, Roche, Pfizer, Sanofi, Amgen, Janssen, Takeda and research grants from MSD and Merck. M.P. has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome and Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by M.P. with payments made to his institution: Böhringer Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline and AbbVie. The remaining authors declare no competing interests.
Figures
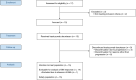
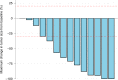

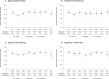

References
-
- Sanna G, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res. 2007;27:2865–2869. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

