Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis
- PMID: 35941646
- PMCID: PMC9358061
- DOI: 10.1186/s12985-022-01858-3
Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis
Abstract
Background: Immunocompromised (IC) patients are at higher risk of more severe COVID-19 infections than the general population. Special considerations should be dedicated to such patients. We aimed to investigate the efficacy of COVID-19 vaccines based on the vaccine type and etiology as well as the necessity of booster dose in this high-risk population.
Materials and methods: We searched PubMed, Web of Science, and Scopus databases for observational studies published between June 1st, 2020, and September 1st, 2021, which investigated the seroconversion after COVID-19 vaccine administration in adult patients with IC conditions. For investigation of sources of heterogeneity, subgroup analysis and sensitivity analysis were conducted. Statistical analysis was performed using R software.
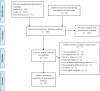
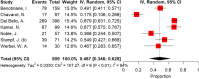
Results: According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, we included 81 articles in the meta-analysis. The overall crude prevalence of seroconversion after the first (n: 7460), second (n: 13,181), and third (n: 909, all population were transplant patients with mRNA vaccine administration) dose administration was 26.17% (95% CI 19.01%, 33.99%, I2 = 97.1%), 57.11% (95% CI: 49.22%, 64.83%, I2 = 98.4%), and 48.65% (95% CI: 34.63%, 62.79%, I2 = 94.4%). Despite the relatively same immunogenicity of mRNA and vector-based vaccines after the first dose, the mRNA vaccines induced higher immunity after the second dose. Regarding the etiologic factor, transplant patients were less likely to develop immunity after both first and second dose rather than patients with malignancy (17.0% vs 37.0% after first dose, P = 0.02; 38.3% vs 72.1% after second dose, P < 0.001) or autoimmune disease (17.0% vs 36.4%, P = 0.04; 38.3% vs 80.2%, P < 0.001). To evaluate the efficacy of the third dose, we observed an increasing trend in transplant patients after the first (17.0%), second (38.3%), and third (48.6%) dose.
Conclusion: The rising pattern of seroconversion after boosting tends to be promising. In this case, more attention should be devoted to transplant patients who possess the lowest response rate.
Keywords: Autoimmune; COVID-19; Efficacy; Immunocompromised patient; Malignancy; SARS-CoV-2; Transplantation; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

