A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis
- PMID: 35941676
- PMCID: PMC9358905
- DOI: 10.1186/s13075-022-02877-9
A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis
Abstract
Objective: To assess the efficacy and safety of infliximab versus placebo in the treatment of patients with juvenile-onset spondyloarthritis (JoSpA).
Methods: Phase III, randomized, double-blind, placebo-controlled trial of 12 weeks that included patients ≤ 18 years old with JoSpA not responding to nonsteroidal anti-inflammatory drugs, sulfasalazine, or methotrexate. Patients were randomly assigned 1:1 to the infusion of infliximab 5mg/kg or placebo; completers entered then an open-label extension (OLE) period of 42 weeks. The primary endpoint was the number of active joints. Secondary outcomes included the assessment of disease activity, tender entheses, spinal mobility, serum C-reactive protein (CRP), the Bath Ankylosing Spondylitis Disease Activity and Functional Index, and the Childhood Health Assessment Questionnaire (CHAQ).
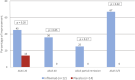
Results: We randomized 12 patients to infliximab and 14 to placebo. No significant differences were found between groups at baseline. At week 12, the mean number of active joints was 1.4 (SD 2.4) in the infliximab group and 4.1 (SD 3.0) in the placebo group (p = 0.0002). A repeated-measures mixed model analysis that included all endpoints in the study demonstrated sustained favourable outcomes of infliximab for active joints, tender joints, swollen joints, and tender enthesis counts, as well as for CHAQ and CRP (p < 0.01). Adverse events were more frequent in the infliximab group, including infections and infusion reactions, but none of them was serious.
Conclusion: Infliximab is efficacious for patients with JoSpA with an inadequate response to conventional treatment. No serious adverse events with the use of infliximab were observed.
Keywords: Active joint counts; Infliximab; Juvenile SpA; Open-label study; Randomized trial; Spondyloarthritis.
© 2022. The Author(s).
Conflict of interest statement
Dr. Rubén Burgos Vargas received funding from Schering Plough, Mexico, to complete the data collection of this study. None of the other authors received any compensation from this or any other pharmaceutical company for the analysis or interpretation of the results.
Figures




References
-
- Burgos-Vargas R, Clark P. Axial involvement in the seronegative enthesopathy and arthropathy syndrome and its progression to ankylosing spondylitis. J Rheumatol. 1989;16(2):192–197. - PubMed
-
- Cabral DA, Oen KG, Petty RE. SEA syndrome revisited: a longterm followup of children with a syndrome of seronegative enthesopathy and arthropathy. J Rheumatol. 1992;19(8):1282–1285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

