Initiating Intramuscular Depot Medroxyprogesterone Acetate Increases Frequencies of Th17-like Human Immunodeficiency Virus Target Cells in the Genital Tract of Women in South Africa: A Randomized Trial
- PMID: 35941737
- PMCID: PMC9710690
- DOI: 10.1093/cid/ciac284
Initiating Intramuscular Depot Medroxyprogesterone Acetate Increases Frequencies of Th17-like Human Immunodeficiency Virus Target Cells in the Genital Tract of Women in South Africa: A Randomized Trial
Erratum in
-
Correction to: Impact of Point-of-Care Testing on the Management of Sexually Transmitted Infections in South Africa: Evidence from the HVTN702 Human Immunodeficiency Virus Vaccine Trial.Clin Infect Dis. 2023 Jan 6;76(1):182. doi: 10.1093/cid/ciac898. Clin Infect Dis. 2023. PMID: 36450653 Free PMC article. No abstract available.
Abstract
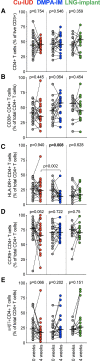
Background: Cervicovaginal CD4+ T cells are preferential targets for human immunodeficiency virus (HIV) infection and have consequently been used as a proxy measure for HIV susceptibility. The ECHO randomized trial offered a unique opportunity to consider the association between contraceptives and Th17-like cells within a trial designed to evaluate HIV risk. In a mucosal substudy of the ECHO trial, we compared the impact of initiating intramuscular depot medroxyprogesterone acetate (DMPA-IM), copper-IUD, and the levonorgestrel (LNG) implant on cervical T cells.
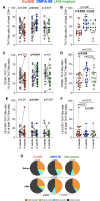
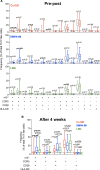
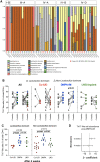
Methods: Cervical cytobrushes from 58 women enrolled in the ECHO trial were collected at baseline and 1 month after contraceptive initiation. We phenotyped cervical T cells using multiparameter flow cytometry, characterized the vaginal microbiome using 16s sequencing, and determined proteomic signatures associated with Th17-like cells using mass spectrometry.
Results: Unlike the LNG implant or copper-IUD, DMPA-IM was associated with higher frequencies of cervical Th17-like cells within 1 month of initiation (P = .012), including a highly susceptible, activated population co-expressing CD38, CCR5, and α4β7 (P = .003). After 1 month, women using DMPA-IM also had more Th17-like cells than women using the Cu-IUD (P = .0002) or LNG implant (P = .04). Importantly, in women using DMPA-IM, proteomic signatures signifying enhanced mucosal barrier function were associated with the increased abundance of Th17-like cells. We also found that a non-Lactobacillus-dominant microbiome at baseline was associated with more Th17-like cells post-DMPA-IM (P = .03), although this did not influence barrier function.
Conclusions: Our data suggest that DMPA-IM-driven accumulation of HIV-susceptible Th17-like cells might be counteracted by their role in maintaining mucosal barrier integrity.
Clinical trials registration: NCT02550067.
Keywords: HIV risk; Th17 cells; hormonal contraception; mucosal barrier integrity.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J.-A. S. P. reports the following grants or contracts all paid to the author’s institution and unrelated to this work: Bill and Melinda Gates Foundation (BMGF) Vaginal Microbiome Research Consortium (VMRC) Planning Grant (Principal Investigator [PI]: Dr Jo-Ann Passmore. Co-PI: Dr Leila Mansoor; Co-Investigators: Nigel Garrett, Cheryl Baxter, Sinaye Ngcapu, Lenine Liebenberg, Aida Sivro, Brian Kullin, Anna Happel. US $512 088 for 12 months); European and Developing Countries Clinical Trials Partnership (EDCTP) RIA2020I (3297) for project entitled “GIFT for HIV Prevention” (Passmore, Co-PI: Masson (Burnett Institute, Australia); Co-Investigators: Suzanna Francis and Katharina Kranzer (The London School of Hygiene & Tropical Medicine, UK), Janneke van der Wijgert (University Medical Center, Netherlands), Tania Crucitti (Institute Pasteur Madegascar, Madagascar), David Anderson (Burnet Institute, Australia), Ayako Honda (Sophia University, Japan), Chido Dziva-Chikwari (The Organization for Public Health Interventions and Development, Zimbabwe), Katherine Gill (Desmond Tutu Health Foundation, South Africa) (Euro 3 508 462 for 36 months); BMGF Calestous Juma Scientific Leadership Award for project entitled “VMRC4Africa” (PI: Passmore. US $1 million over 5 years); and Medical Research Council Strategic Health Innovation Partnerships (South Africa), Genital inflammation test for females (GIFT) (PIs: Dr Jo-Ann Passmore and Dr Lindi Masson, ZAR 5 million per year for 4 years). J.-A. S. P. also reports a patent granted in 2022 with no payment made (Method for diagnosing an inflammatory condition in the female genital tract; PCT/IB2014/065740; EP3063542B1); and unpaid participation on a Data Safety Monitoring Board (DSMB) or Advisory Board for a phase 2 placebo-controlled randomized trial of LACTIN-V (Lactobacillus crispatus CTV-05) among women at high risk of HIV acquisition in Durban, South Africa (Chair DSMB; Cohen et al National Institute of Child Health and Human Development [NICHD] grant 1R01HD098978). R. H. reports grants or contracts unrelated to this work and paid to the author’s institution from the NICHD. H. J. reports support for attending meetings and/or travel from World Vaccine Congress. S. E. B. reports grants or contracts unrelated to this work from the National Institutes of Health (NIH R01 HD089831): Effects of Hormonal Contraceptives on Genital Immunity and HIV Susceptibility. B. P. B. reports support for attending meetings and/or travel (NIH R01HD089831). J. M. H. reports grants to institution unrelated to this work from the NIH, USAID, and BMGF; and employment (with stocks and stock options) with Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

