Online Monitoring of the Concentrations of Amorphous and Crystalline Mesoscopic Species Present in Solution
- PMID: 35942122
- PMCID: PMC9354028
- DOI: 10.1021/acs.cgd.2c00577
Online Monitoring of the Concentrations of Amorphous and Crystalline Mesoscopic Species Present in Solution
Abstract
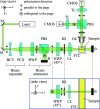
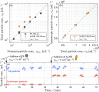
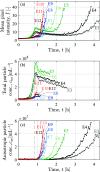
Despite the growing evidence for the existence of amorphous mesoscopic species in a solution and their crucial roles in crystallization, there has been the lack of a suitable method to measure the time-resolved concentrations of amorphous and crystalline mesospecies in a lab-scale stirred reactor. This has limited experimental investigations to understand the kinetics of amorphous and crystalline mesospecies formation in stirred solutions and made it challenging to measure the crystal nucleation rate directly. Here, we used depolarized light sheet microscopy to achieve time-resolved measurements of amorphous and crystalline mesospecies concentrations in solutions at varying temperatures. After demonstrating that the concentration measurement method is reasonably accurate, precise, and sensitive, we utilized this method to examine mesospecies formation both in a mixture of two miscible liquids and in an undersaturated solution of dl-valine, thus revealing the importance of a temperature change in the formation of metastable and amorphous mesospecies as well as the reproducibility of the measurements. Moreover, we used the presented method to monitor both mesospecies formation and crystal nucleation in dl-valine solutions at four different levels of supersaturation, while achieving the direct measurement of the crystal nucleation rates in stirred solutions. Our results show that, as expected, the inherent variability in nucleation originating from its stochastic nature reduces with increasing supersaturation, and the dependence of the measured nucleation rate on supersaturation is in reasonable agreement with that predicted by the classical nucleation theory.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Population and size distribution of solute-rich mesospecies within mesostructured aqueous amino acid solutions.Faraday Discuss. 2013;167:425-40. doi: 10.1039/c3fd00066d. Faraday Discuss. 2013. PMID: 24640504
-
Size-Independent Nucleation and Growth Model of Potassium Sulfate from Supersaturated Solution Produced by Stirred Crystallization.Molecules. 2023 Dec 26;29(1):141. doi: 10.3390/molecules29010141. Molecules. 2023. PMID: 38202723 Free PMC article.
-
Multiple pathways of crystal nucleation in an extremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet.Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):13618-13623. doi: 10.1073/pnas.1604938113. Epub 2016 Oct 24. Proc Natl Acad Sci U S A. 2016. PMID: 27791068 Free PMC article.
-
Nucleation precursors in protein crystallization.Acta Crystallogr F Struct Biol Commun. 2014 Mar;70(Pt 3):271-82. doi: 10.1107/S2053230X14002386. Epub 2014 Feb 20. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24598910 Free PMC article. Review.
-
The two-step mechanism of nucleation of crystals in solution.Nanoscale. 2010 Nov;2(11):2346-57. doi: 10.1039/c0nr00628a. Epub 2010 Oct 8. Nanoscale. 2010. PMID: 20936214 Review.
Cited by
-
Conceptual Validation of Stochastic and Deterministic Methods To Estimate Crystal Nucleation Rates.Cryst Growth Des. 2022 Dec 29;23(2):899-914. doi: 10.1021/acs.cgd.2c01133. eCollection 2023 Feb 1. Cryst Growth Des. 2022. PMID: 36747576 Free PMC article.
-
Rationalizing the Influence of Solvent on the Nucleation of Griseofulvin through Classical and Nonclassical Pathways.Cryst Growth Des. 2025 Jun 3;25(13):4713-4724. doi: 10.1021/acs.cgd.5c00206. eCollection 2025 Jul 2. Cryst Growth Des. 2025. PMID: 40625785 Free PMC article.
References
-
- Gallego-Urrea J. A.; Tuoriniemi J.; Hassellöv M. Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. TrAC Trends Anal. Chem. 2011, 30, 473–483. 10.1016/j.trac.2011.01.005. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
