MicroRNA-147a Targets SLC40A1 to Induce Ferroptosis in Human Glioblastoma
- PMID: 35942174
- PMCID: PMC9356897
- DOI: 10.1155/2022/2843990
MicroRNA-147a Targets SLC40A1 to Induce Ferroptosis in Human Glioblastoma
Abstract
Objective: Glioblastoma is one of the most common malignant tumors in the brain, and these glioblastoma patients have very poor prognosis. Ferroptosis is involved in the progression of various tumors, including the glioblastoma. This study aims to determine the involvement of microRNA (miR)-147a in regulating ferroptosis of glioblastoma in vitro.
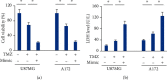
Methods: Human glioblastoma cell lines were transfected with the inhibitor, mimic and matched negative controls of miR-147a in the presence or absence of ferroptotic inducers. To knock down the endogenous solute carrier family 40 member 1 (SLC40A1), cells were transfected with the small interfering RNA against SLC40A1. In addition, cells with or without the miR-147a mimic treatment were also incubated with temozolomide (TMZ) to investigate whether miR-147a overexpression could sensitize human glioblastoma cells to TMZ chemotherapy in vitro.
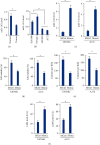
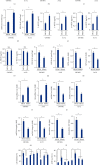
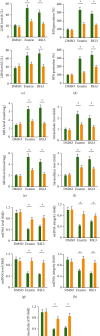
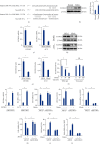
Results: We found that miR-147a level was decreased in human glioblastoma tissues and cell lines and that the miR-147a mimic significantly suppressed the growth of glioblastoma cells in vitro. In addition, miR-147a expression was elevated in human glioblastoma cells upon erastin or RSL3 stimulation. Treatment with the miR-147a mimic significantly induced ferroptosis of glioblastoma cells, and the ferroptotic inhibitors could block the miR-147a mimic-mediated tumor suppression in vitro. Conversely, the miR-147a inhibitor prevented erastin- or RSL3-induced ferroptosis and increased the viability of glioblastoma cells in vitro. Mechanistically, we determined that miR-147a directly bound to the 3'-untranslated region of SLC40A1 and inhibited SLC40A1-mediated iron export, thereby facilitating iron overload, lipid peroxidation, and ferroptosis. Furthermore, miR-147a mimic-treated human glioblastoma cells exhibited higher sensitivity to TMZ chemotherapy than those treated with the mimic control in vitro.
Conclusion: We for the first time determine that miR-147a targets SLC40A1 to induce ferroptosis in human glioblastoma in vitro.
Copyright © 2022 Peng Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Wang B., Peng F., Huang W., et al. Rational drug design, synthesis, and biological evaluation of novel chiral tetrahydronaphthalene-fused spirooxindole as MDM2-CDK4 dual inhibitor against glioblastoma. Acta Pharmaceutica Sinica B . 2020;10(8):1492–1510. doi: 10.1016/j.apsb.2019.12.013. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

