Transmembrane Protein ANTXR1 Regulates γ-Globin Expression by Targeting the Wnt/ β-Catenin Signaling Pathway
- PMID: 35942209
- PMCID: PMC9356848
- DOI: 10.1155/2022/8440422
Transmembrane Protein ANTXR1 Regulates γ-Globin Expression by Targeting the Wnt/ β-Catenin Signaling Pathway
Abstract
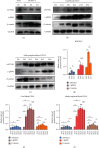
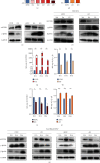
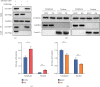
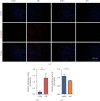
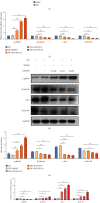
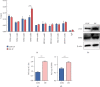
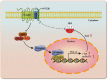
Reactivation of fetal hemoglobin (HbF, α2γ2) alleviates clinical symptoms in patients with β-thalassemia and sickle cell disease, although the regulatory mechanisms of γ-globin expression have not yet been fully elucidated. Recent studies found that interfering with the expression of the membrane protein ANTXR1 gene upregulated γ-globin levels. However, the exact mechanism by which ANTXR1 regulates γ-globin levels remains unclear. Our study showed that overexpression and knockdown of ANTXR1 in K562, cord blood CD34+, and HUDEP-2 cells decreased and increased γ-globin expression, respectively. ANTXR1 regulates the reactivation of fetal hemoglobin (HbF, α2γ2) in K562, cord blood CD34+, and adult peripheral blood CD34+ cells through interaction with LRP6 to promote the nuclear entry of β-catenin and activate the Wnt/β-catenin signaling pathway. The overexpression or knockdown of ANTXR1 on γ-globin and Wnt/β-catenin signaling in K562 cells was reversed by the inhibitor XAV939 and the activator LiCl, respectively, where XAV939 inhibits the transcription of β-catenin in the Wnt pathway, but LiCl inhibits GSK3-β. We also showed that the binding ability of the rank4 site in the transcriptional regulatory region of the SOX6 gene to c-Jun was significantly increased after overexpression of ANTXR1 in K562 cells. SOX6 protein expression was increased significantly after overexpression of the c-Jun gene, indicating that the transcription factor c-Jun initiated the transcription of SOX6, thereby silencing γ-globin. Our findings may provide a new intervention target for the treatment of β-hemoglobinopathies.
Copyright © 2022 Tingting Jin et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Hydroxyurea-inducible SAR1 gene acts through the Giα/JNK/Jun pathway to regulate γ-globin expression.Blood. 2014 Aug 14;124(7):1146-56. doi: 10.1182/blood-2013-10-534842. Epub 2014 Jun 9. Blood. 2014. PMID: 24914133 Free PMC article.
-
ANTXR1 Regulates Erythroid Cell Proliferation and Differentiation through wnt/β-Catenin Signaling Pathway In Vitro and in Hematopoietic Stem Cell.Dis Markers. 2022 Aug 27;2022:1226697. doi: 10.1155/2022/1226697. eCollection 2022. Dis Markers. 2022. PMID: 36065334 Free PMC article.
-
KLF1 Knockdown Differentially Regulates γ-Globin Expression: Inhibition in K562 Cells but Reactivation in β-Thalassemia Major Erythrocytes with Erythropoiesis Disruption.Hemoglobin. 2025 Jul;49(4):244-251. doi: 10.1080/03630269.2025.2514142. Epub 2025 Jul 6. Hemoglobin. 2025. PMID: 40619594
-
Hemoglobin genetics: recent contributions of GWAS and gene editing.Hum Mol Genet. 2016 Oct 1;25(R2):R99-R105. doi: 10.1093/hmg/ddw170. Epub 2016 Jun 23. Hum Mol Genet. 2016. PMID: 27340226 Free PMC article. Review.
-
Pharmacologic induction of fetal hemoglobin production.Hematol Oncol Clin North Am. 2010 Dec;24(6):1131-44. doi: 10.1016/j.hoc.2010.08.001. Epub 2010 Sep 29. Hematol Oncol Clin North Am. 2010. PMID: 21075284 Review.
Cited by
-
Differential MicroRNA Profiles Associated With the Hydroxyurea-Inducible SAR1A Gene.J Hematol. 2025 Jun;14(3):158-163. doi: 10.14740/jh2021. Epub 2025 Apr 22. J Hematol. 2025. PMID: 40501517 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

