Epoxides: Small Rings to Play with under Asymmetric Organocatalysis
- PMID: 35942279
- PMCID: PMC9354533
- DOI: 10.1021/acsorginorgau.2c00009
Epoxides: Small Rings to Play with under Asymmetric Organocatalysis
Abstract
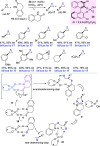
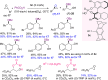
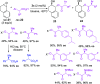
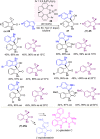
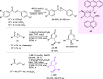
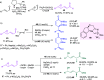
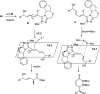
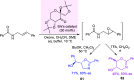
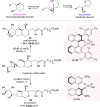
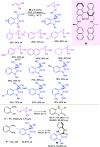
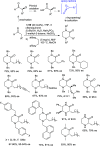
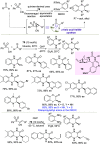
Optically pure epoxides are recognized as highly valuable products and key intermediates, useful in different areas from pharmaceutical and agrochemical industries to natural product synthesis and materials science. The predictable fate of the ring-opening process, in terms of stereoselectivity and often of regioselectivity, enables useful functional groups to be installed at vicinal carbon atoms in a desired manner. In this way, products of widespread utility either for synthetic applications or as final products can be obtained. The advent of asymmetric organocatalysis provided a new convenient tool, not only for their preparation but also for the elaboration of this class of heterocycles. In this review, we focus on recent developments of stereoselective organocatalytic ring-opening reactions of meso-epoxides, kinetic resolution of racemic epoxides, and Meinwald-type rearrangement. Examples of asymmetric organocatalytic processes toward specific synthetic targets, which include ring opening of an epoxide intermediate, are also illustrated.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

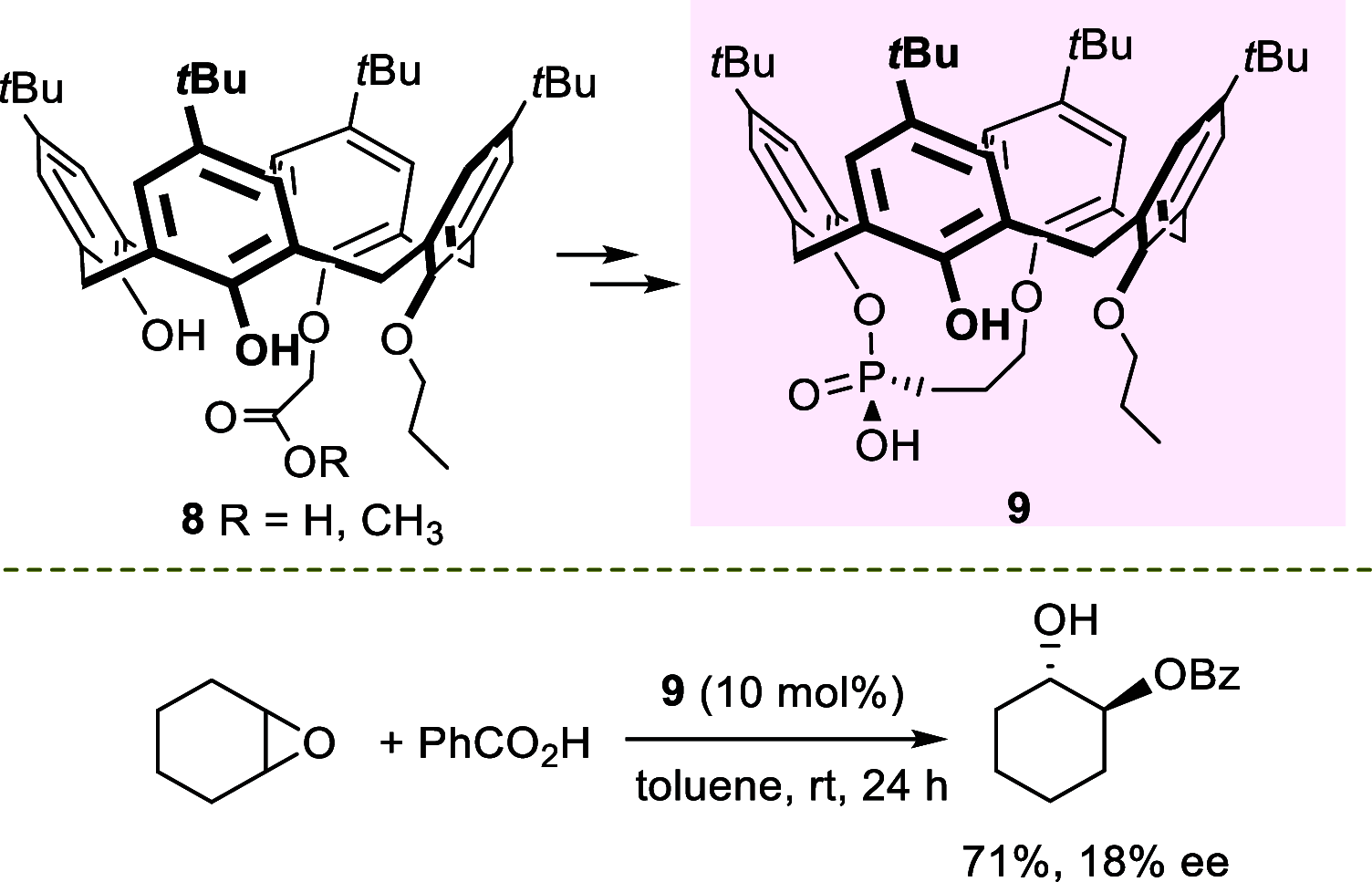



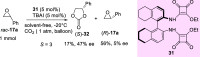
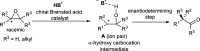

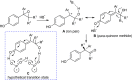
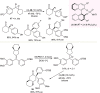




References
-
- Sheldon R. A.Chirotechnology; Marcel Dekker: New York, 1993.
-
- Blaser H. U., Federsel H.-J., Eds. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010.
-
- Rouhi A. M. Chiral business. Chem. Eng. News 2003, 81, 45–55. 10.1021/cen-v081n018.p045. - DOI
Publication types
LinkOut - more resources
Full Text Sources
