Impact of moderate or severe left ventricular outflow tract calcification on clinical outcomes of patients with severe aortic stenosis undergoing transcatheter aortic valve implantation with self- and balloon-expandable valves: a post hoc analysis from the SOLVE-TAVI trial
- PMID: 35942626
- PMCID: PMC11064680
- DOI: 10.4244/EIJ-D-22-00156
Impact of moderate or severe left ventricular outflow tract calcification on clinical outcomes of patients with severe aortic stenosis undergoing transcatheter aortic valve implantation with self- and balloon-expandable valves: a post hoc analysis from the SOLVE-TAVI trial
Abstract
Background: Left ventricular outflow tract (LVOT) calcification has been associated with worse outcomes in patients undergoing transcatheter aortic valve implantation (TAVI) and may influence the selection of prosthetic valve type.
Aims: We aimed to evaluate the impact of LVOT calcification on outcomes after TAVI with a self-expanding valve (SEV) versus a balloon-expandable valve (BEV).
Methods: Patients of the SOLVE-TAVI trial, randomised to Edwards SAPIEN 3 or Medtronic Evolut R, were divided according to LVOT calcification into no/mild (≤1 calcium nodule extending <5 mm and covering <10% of the LVOT perimeter) and moderate/severe LVOT calcification groups. The primary endpoint was a composite of death, stroke, moderate/severe paravalvular regurgitation, permanent pacemaker implantation and annulus rupture at 30 days. Additional endpoints included all-cause and cardiovascular mortality at 1 year.
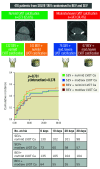
Results: Out of 416 eligible patients, moderate/severe LVOT calcification was present in 143 (34.4%). Moderate/severe LVOT calcification was associated with significantly longer fluoroscopy time and higher rates of pre- and post-dilation. Regardless of the LVOT calcification group, there was no significant difference in the primary endpoint associated with the valve type (no/mild LVOT calcification group: SEV 25.0% vs BEV 27.0%; hazard ratio [HR] 1.10, 95% confidence interval [95% CI]: 0.68-1.73; p=0.73 and moderate/severe LVOT calcification group: SEV 25.0% vs BEV 19.4%; HR 0.76, 95% CI: 0.38-1.61; p=0.49), no significant interaction between LVOT calcification and valve type (pint=0.29) and no differences between SEV vs BEV within LVOT calcification groups regarding 1-year all-cause and cardiovascular mortality.
Conclusions: Moderate/severe LVOT calcification was associated with longer fluoroscopy time and an increased need for pre- and post-dilation, but not with a higher incidence of early and mid-term adverse clinical outcomes, regardless of valve type. (ClinicalTrials.gov: NCT02737150).
Conflict of interest statement
D. Holzhey is advisor/proctor for Edwards Lifesciences and Medtronic. U. Landmesser has received lecture and advisory honoraria from Abbott and Boston Scientific (outside the submitted work). M.A. Borger received consulting fees and honoraria paid to his hospital on his behalf from Edwards Lifesciences, Medtronic, CryoLife, and Abbott. The other authors have no conflicts of interest to declare.
Figures
References
-
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG PARTNER 2 Investigators. Transcatheter or Surgical Aortic Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. - PubMed
-
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–705. - PubMed
-
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706–15. - PubMed
-
- Hayashida K, Bouvier E, Lefèvre T, Hovasse T, Morice MC, Chevalier B, Romano M, Garot P, Farge A, Donzeau-Gouge P, Cormier B. Potential mechanism of annulus rupture during transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;82:E742–6. - PubMed
-
- Jochheim D, Deseive S, Gschwendtner S, Bischoff B, Jochheim S, Hausleiter S, Zadrozny M, Baquet M, Tesche C, Massberg S, Mehilli J, Hausleiter J. Impact of severe left ventricular outflow tract calcification on device failure and short-term mortality in patients undergoing TAVI. J Cardiovasc Comput Tomogr. 2020;14:36–41. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

