Physical activity and exercise training in cystic fibrosis
- PMID: 35943025
- PMCID: PMC9361297
- DOI: 10.1002/14651858.CD002768.pub5
Physical activity and exercise training in cystic fibrosis
Abstract
Background: Physical activity (including exercise) may form an important part of regular care for people with cystic fibrosis (CF). This is an update of a previously published review.
Objectives: To assess the effects of physical activity interventions on exercise capacity by peak oxygen uptake, lung function by forced expiratory volume in one second (FEV1), health-related quality of life (HRQoL) and further important patient-relevant outcomes in people with cystic fibrosis (CF).
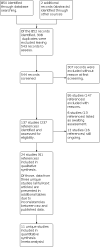
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. The most recent search was on 3 March 2022. We also searched two ongoing trials registers: clinicaltrials.gov, most recently on 4 March 2022; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), most recently on 16 March 2022. SELECTION CRITERIA: We included all randomised controlled trials (RCTs) and quasi-RCTs comparing physical activity interventions of any type and a minimum intervention duration of two weeks with conventional care (no physical activity intervention) in people with CF.
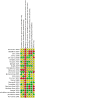
Data collection and analysis: Two review authors independently selected RCTs for inclusion, assessed methodological quality and extracted data. We assessed the certainty of the evidence using GRADE. MAIN RESULTS: We included 24 parallel RCTs (875 participants). The number of participants in the studies ranged from nine to 117, with a wide range of disease severity. The studies' age demographics varied: in two studies, all participants were adults; in 13 studies, participants were 18 years and younger; in one study, participants were 15 years and older; in one study, participants were 12 years and older; and seven studies included all age ranges. The active training programme lasted up to and including six months in 14 studies, and longer than six months in the remaining 10 studies. Of the 24 included studies, seven implemented a follow-up period (when supervision was withdrawn, but participants were still allowed to exercise) ranging from one to 12 months. Studies employed differing levels of supervision: in 12 studies, training was supervised; in 11 studies, it was partially supervised; and in one study, training was unsupervised. The quality of the included studies varied widely. This Cochrane Review shows that, in studies with an active training programme lasting over six months in people with CF, physical activity probably has a positive effect on exercise capacity when compared to no physical activity (usual care) (mean difference (MD) 1.60, 95% confidence interval (CI) 0.16 to 3.05; 6 RCTs, 348 participants; moderate-certainty evidence). The magnitude of improvement in exercise capacity is interpreted as small, although study results were heterogeneous. Physical activity interventions may have no effect on lung function (forced expiratory volume in one second (FEV1) % predicted) (MD 2.41, 95% CI ‒0.49 to 5.31; 6 RCTs, 367 participants), HRQoL physical functioning (MD 2.19, 95% CI ‒3.42 to 7.80; 4 RCTs, 247 participants) and HRQoL respiratory domain (MD ‒0.05, 95% CI ‒3.61 to 3.51; 4 RCTs, 251 participants) at six months and longer (low-certainty evidence). One study (117 participants) reported no differences between the physical activity and control groups in the number of participants experiencing a pulmonary exacerbation by six months (incidence rate ratio 1.28, 95% CI 0.85 to 1.94) or in the time to first exacerbation over 12 months (hazard ratio 1.34, 95% CI 0.65 to 2.80) (both high-certainty evidence); and no effects of physical activity on diabetic control (after 1 hour: MD ‒0.04 mmol/L, 95% CI ‒1.11 to 1.03; 67 participants; after 2 hours: MD ‒0.44 mmol/L, 95% CI ‒1.43 to 0.55; 81 participants; moderate-certainty evidence). We found no difference between groups in the number of adverse events over six months (odds ratio 6.22, 95% CI 0.72 to 53.40; 2 RCTs, 156 participants; low-certainty evidence). For other time points (up to and including six months and during a follow-up period with no active intervention), the effects of physical activity versus control were similar to those reported for the outcomes above. However, only three out of seven studies adding a follow-up period with no active intervention (ranging between one and 12 months) reported on the primary outcomes of changes in exercise capacity and lung function, and one on HRQoL. These data must be interpreted with caution. Altogether, given the heterogeneity of effects across studies, the wide variation in study quality and lack of information on clinically meaningful changes for several outcome measures, we consider the overall certainty of evidence on the effects of physical activity interventions on exercise capacity, lung function and HRQoL to be low to moderate.
Authors' conclusions: Physical activity interventions for six months and longer likely improve exercise capacity when compared to no training (moderate-certainty evidence). Current evidence shows little or no effect on lung function and HRQoL (low-certainty evidence). Over recent decades, physical activity has gained increasing interest and is already part of multidisciplinary care offered to most people with CF. Adverse effects of physical activity appear rare and there is no reason to actively discourage regular physical activity and exercise. The benefits of including physical activity in an individual's regular care may be influenced by the type and duration of the activity programme as well as individual preferences for and barriers to physical activity. Further high-quality and sufficiently-sized studies are needed to comprehensively assess the benefits of physical activity and exercise in people with CF, particularly in the new era of CF medicine.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
HH has received financial compensation for travel and accommodation or free meeting participation (or both) at the European Cystic Fibrosis Society conference and the North American Cystic Fibrosis Conference for chairing or presenting at sessions focusing on exercise in cystic fibrosis. For writing an educational booklet on exercise in cystic fibrosis, HH has received money from Novartis. HH is also the lead investigator on one of the studies included in this review (Hebestreit 2010). As he is the lead investigator of the international multicentre trial ACTIVATE‐CF (Hebestreit 2022), his institution has received grants from the Mukoviszidose e.V. and a Vertex Innovation Award.
TR was a core study team member of the ACTIVATE‐CF trial (Hebestreit 2022). TR has received financial compensation for chairing and presenting at exercise sessions at the European Cystic Fibrosis Society conference. He has also received financial support (travel, accommodation) from Vifor Pharma Switzerland to participate at the European Cystic Fibrosis Society and European Respiratory Society conference.
SK is the lead investigator on one of the studies included in the review (Kriemler 2013), and was a core team member of the ACTIVATE‐CF trial (Hebestreit 2022)
SJN declares no known potential conflicts of interest.
SS declares no known potential conflicts of interest.
Figures














































Update of
-
Physical exercise training for cystic fibrosis.Cochrane Database Syst Rev. 2017 Nov 1;11(11):CD002768. doi: 10.1002/14651858.CD002768.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Aug 9;8:CD002768. doi: 10.1002/14651858.CD002768.pub5. PMID: 29090734 Free PMC article. Updated.
Similar articles
-
Exercise versus airway clearance techniques for people with cystic fibrosis.Cochrane Database Syst Rev. 2022 Jun 22;6(6):CD013285. doi: 10.1002/14651858.CD013285.pub2. Cochrane Database Syst Rev. 2022. PMID: 35731672 Free PMC article.
-
Physical exercise training for cystic fibrosis.Cochrane Database Syst Rev. 2017 Nov 1;11(11):CD002768. doi: 10.1002/14651858.CD002768.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Aug 9;8:CD002768. doi: 10.1002/14651858.CD002768.pub5. PMID: 29090734 Free PMC article. Updated.
-
Macrolide antibiotics (including azithromycin) for cystic fibrosis.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD002203. doi: 10.1002/14651858.CD002203.pub5. Cochrane Database Syst Rev. 2024. PMID: 38411248 Free PMC article.
-
Nebulised hypertonic saline for cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 14;6(6):CD001506. doi: 10.1002/14651858.CD001506.pub5. Cochrane Database Syst Rev. 2023. PMID: 37319354 Free PMC article.
-
Nebulised hypertonic saline for cystic fibrosis.Cochrane Database Syst Rev. 2018 Sep 27;9(9):CD001506. doi: 10.1002/14651858.CD001506.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jun 14;6:CD001506. doi: 10.1002/14651858.CD001506.pub5. PMID: 30260472 Free PMC article. Updated.
Cited by
-
Telemedicine and Its Application in Cystic Fibrosis.J Pers Med. 2023 Jun 25;13(7):1041. doi: 10.3390/jpm13071041. J Pers Med. 2023. PMID: 37511654 Free PMC article. Review.
-
Response to Comment on: "Low Cardiorespiratory Fitness Post-COVID-19: A Narrative Review".Sports Med. 2023 Dec;53(12):2531-2532. doi: 10.1007/s40279-023-01922-0. Epub 2023 Sep 8. Sports Med. 2023. PMID: 37682410 Review. No abstract available.
-
Longitudinal changes in habitual physical activity in adult people with cystic fibrosis in the presence or absence of treatment with elexacaftor/tezacaftor/ivacaftor.Front Sports Act Living. 2024 Feb 23;6:1284878. doi: 10.3389/fspor.2024.1284878. eCollection 2024. Front Sports Act Living. 2024. PMID: 38463712 Free PMC article.
-
A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy.Nutrients. 2025 Jul 25;17(15):2439. doi: 10.3390/nu17152439. Nutrients. 2025. PMID: 40806025 Free PMC article.
-
Effects of a Tailored Home-Based Exercise Program, "KidMove", on Children with Cystic Fibrosis: A Quasi-Experimental Study.Healthcare (Basel). 2024 Dec 24;13(1):4. doi: 10.3390/healthcare13010004. Healthcare (Basel). 2024. PMID: 39791611 Free PMC article.
References
References to studies included in this review
Alexander 2019 {published data only}
-
- Alexander E, Wilson C, Kapur N, Brookes D. Is telehealth as effective as face to face therapy for delivery of whole body vibration training (WBVT) as an adjunct to physiotherapy in children and young people with cystic fibrosis (CF)? Respirology 2019;24:135. [CFGD REGISTER: MH178]
Beaudoin 2017 {published data only}
-
- Beaudoin N, Bouvet GF, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Combined exercise training improves glycemic control in adults with cystic fibrosis. Medicine and Science in Sports and Exercise 2017;49(2):231-7. [CENTRAL: 1199954] [CFGD REGISTER: CO62b] [PMID: ] - PubMed
-
- Beaudoin N, Bouvet GF, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Combined exercise training improves glycemic control in adults with cystic fibrosis. Medicine and Science in Sports and Exercise 2017;49(2):231-7.Supplemental digital content. [CFGD REGISTER: CO62c] - PubMed
-
- Beaudoin N, Bouvet GF, Rabasa-Lhoret R, Berthiaume Y. Effects of a partially supervised combined exercise program on glycemic control in cystic fibrosis: pilot study. Pediatric Pulmonology 2015;50(Suppl 41):367. [ABSTRACT NO: 466] [CENTRAL: 1092169] [CFGD REGISTER: CO62a] [EMBASE: 72081745]
-
- NCT02127957. Effects of an exercise program among CF patients with dysglycemia (FKEX). clinicaltrials.gov/ct2/show/NCT02127957 (first received 1 May 2014).
Carr 2018 {published data only}
-
- Carr SB, Ronan P, Lorenc A, Mian A, Madge SL, Robinson N. Children and adults Tai Chi study (CF-CATS2): a randomised controlled feasibility study comparing internet-delivered with face-to-face Tai Chi lessons in cystic fibrosis. ERJ Open Research 2018;4(4):pii: 00042-2018. [CENTRAL: CN-01925903] [CFGD REGISTER: PE243f] [DOI: 10.1183/23120541.00042-2018] [EMBASE: 625801573] - DOI - PMC - PubMed
-
- Carr SB, Ronan P, Robinson N, Agent P, Mian A, Madge S. A randomised controlled trial of Tai Chi in cystic fibrosis. Pediatric Pulmonology 2016;51:376. [ABSTRACT NO: 480] [CENTRAL: 1212611] [CFGD REGISTER: PE243c] [EMBASE: 612358588]
-
- Lorenc A, Ronan P, Mian A, Madge S, Carr SB, Agent P, et al. Cystic fibrosis-Children and adults Tai Chi study (CF CATS2): can Tai Chi improve symptoms and quality of life for people with cystic fibrosis? Second phase study protocol. Chinese Journal of Integrative Medicine 2015 May 26 [Epub ahead of print]. [CENTRAL: 1343883] [CFGD REGISTER: PE243a] [DOI: 10.1007/s11655-015-2150-1] [PMID: ] - DOI - PubMed
-
- Madge S, Ronan P, Robinson N, Agent P, Mian A, Carr SB. Assessing the benefits of tai chi in people with CF and the feasibility of delivering Tai Chi over the internet (Skype®). Pediatric Pulmonology 2016;51(Suppl 45):370. [ABSTRACT NO: 465] [CENTRAL: 1343884] [CFGD REGISTER: PE243b]
-
- NCT02054377. Cystic fibrosis – children and adults Tai Chi study. clinicaltrials.gov/show/NCT02054377 (first posted 4 February 2014). [CFGD REGISTER: PE243h]
Cerny 1989 {published data only}
-
- Cerny FJ. Relative effects of bronchial drainage and exercise for in-hospital care of patients with cystic fibrosis. Physical Therapy 1989;69(8):633-9. [CFGD REGISTER: PE13] - PubMed
Del Corral 2018 {published data only}
-
- Del Corral T, Cebria I Iranzo MA, Lopez-de-Uralde-Villanueva I, Martinez-Alejos R, Blanco I, Vilaro J. Effectiveness of a home-based active video game programme in young cystic fibrosis patients. Respiration; International Review of Thoracic Diseases 2018;95(2):87-97. [CENTRAL: CN-01643331] [CFGD REGISTER: PE254] [EMBASE: 618880167] [PMID: ] - PubMed
-
- NCT02552043. Video game exercise effectiveness of a domiciliary pulmonary rehabilitation program in cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT02552043 (first received 15 September 2015).
Donadio 2020 {published data only}
-
- Donadio MV, Sanz V, Villa JR, Giron Moreno RM, Cobo F, Diez-Vega I, et al. Effects of exercise with neuromuscular electrical stimulation on peripheral muscle strength, lung function and aerobic fitness in patients with cystic fibrosis: a randomised controlled trial. Journal of Cystic Fibrosis 2020;19(Suppl):S38. [CFGD REGISTER: PE291a]
-
- NCT04153669. Exercise with or without electrical stimulation in cystic fibrosis (part I): effects on physical fitness (ECOMIRIN) [Effects of exercise with or without electrical stimulation in physical fitness in cystic fibrosis: an integrated vision (ECOMIRIN Study)]. clinicaltrials.gov/show/NCT04153669 (first posted 6 November 2019). [CFGD REGISTER: PE291b]
Douglas 2015 {published data only}
-
- Douglas H, Bryon M, Ledger S, Main E. My quality of life or yours? The discrepancies between parent and child reported quality of life scores. Journal of Cystic Fibrosis 2017;16(Suppl 1):S162. [CENTRAL: CN-01445959] [CFGD REGISTER: PE218d]
-
- Douglas H, Ledger S, Main E, Sarria Jaramillo L, Rayner P, Goldman A, et al. INSPIRE-CF: an interim review of participation of children with cystic fibrosis randomised to a weekly supervised exercise intervention. Journal of Cystic Fibrosis 2015;14(Suppl 1):S97. [ABSTRACT NO: 152] [CENTRAL: 1081484] [CFGD REGISTER: PE218a] [EMBASE: 71951805]
-
- Ledger S, Wade A, Douglas H, Sarria L, Rayner P, Goldman A, et al. Interim results for INSPIRE-CF: a 24-month RCT evaluating effects of weekly supervised exercise in children with CF. European Respiratory Journal 2016;48:OA 1493.
-
- Ledger SJ, Douglas H, Sarria Jaramillo L, Rayner P, Aurora P, Main E. INSPIRE-CF: levels of participation of children with cystic fibrosis randomised to a 24-month weekly supervised exercise intervention. Journal of Cystic Fibrosis 2016;15(Suppl 1):S44. [ABSTRACT NO: ePS04.5] [CFGD REGISTER: PE218b] [EMBASE: 614323554]
-
- Ledger SJ, Douglas H, Sarria-Jaramillo L, Rayner P, Goldman A, Giardini A, et al. INSPIRE-CF: a randomised trial evaluating the longitudinal effects of a weekly supervised exercise programme on children with cystic fibrosis. In: World Confederation of Physical Activity Congress; 2017 Jul 2-4; Cape Town, South Africa. Available online at www.abstractstosubmit.com/wcpt2017/abstracts/. 2017.
Güngör 2021 {published data only}
-
- Güngör S, Gencer-Atalay K, Bahar-Özdemir Y, Keniş-Coşkun O, Karadağ-Saygi E. The clinical effects of combining postural exercises with chest physiotherapy in cystic fibrosis: a single-blind, randomized-controlled trial. Turkish Journal of Physical Medicine and Rehabilitation 2021;67(1):91-8. [CFGD REGISTER: PE319b] - PMC - PubMed
-
- NCT03295201. Clinical effects of exercise program added to pulmonary rehabilitation in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03295201 (first received 27 September 2017). [CFGD REGISTER: PE319a]
Gupta 2019 {published data only}CTRI/2013/04/003531
-
- Gupta S, Mukherjee A, Lodha R, Kabra M, Deepak KK, Khadgawat R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian Journal of Pediatrics 2019;86(11):987-94. [CENTRAL: CN-01964575] [CFGD REGISTER: PE279a] [DOI: 10.1007/s12098-019-03019-x] [EMBASE: 2002279681] [PMID: ] - DOI - PubMed
-
- Gupta S, Mukherjee A, Lodha R, Kabra M, Deepak KK, Khadgawat R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian Journal of Pediatrics 2019;86(11):987-94. [CFGD REGISTER: PE279b] [DOI: 10.1007/s12098-019-03019-x] - DOI - PubMed
-
- Gupta S. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003531 (first received 4 April 2013).
Hatziagorou 2019 {published data only}
-
- Hatziagorou E, Giannakoulakos S, Mantsiou C, Kouroukli E, Nousia I, Kampouras A, et al. Does an individualised exercise program improve exercise capacity among young patients with cystic fibrosis? Journal of Cystic Fibrosis 2019;18(Suppl 1):S123. [ABSTRACT NO.: P236] [CENTRAL: CN-01984667] [CFGD REGISTER: PE283]
Hebestreit 2010 {published data only}
-
- Hebestreit A, Kriemler S, Kieser S, Junge S, Heilmann M, Ballmann M, et al. Effects of different conditioning programmes on aerobic capacity in CF. Pediatric Pulmonology 2003;36(Suppl 25):329. [CFGD REGISTER: PE145a]
-
- Hebestreit H, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Long-term effects of a partially supervised conditioning programme in cystic fibrosis. European Respiratory Journal 2010;35(3):578-83. [CFGD REGISTER: PE145b] - PubMed
-
- NCT00231686. Effects of a 6-months physical conditioning program in patients with cystic fibrosis [Effects of a 6-months physical conditioning program on health status and physical activity in youths and young adults with cystic fibrosis – MUKOTRAIN]. clinicaltrials.gov/show/NCT00231686 (first received 4 October 2005). [CFGD REGISTER: PE145d]
Hebestreit 2022 {published data only}
-
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multicenter randomized controlled trial (ACTIVATE-CF). American Journal of Respiratory and Critical Care Medicine 2022;205(3):330-9. [DOI: 10.1164/rccm.202106-1419OC] - DOI - PMC - PubMed
-
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multi-centre, randomised controlled trial (ACTIVATE-CF). Journal of Cystic Fibrosis 2021;20(Suppl):S8. [CFGD REGISTER: PE244e]
-
- Hebestreit H, Lands LC, Alarie N, Schaeff J, Karila C, Orenstein DM, et al. Effects of a partially supervised conditioning programme in cystic fibrosis: an international multi-centre randomised controlled trial (ACTIVATE-CF): study protocol. BMC Pulmonary Medicine 2018;18(1):31. [CENTRAL: CN-01913839] [CFGD REGISTER: PE244b] [EMBASE: 620583585] [PMID: ] - PMC - PubMed
-
- Hebestreit H. How to counsel patients regarding exercise and habitual physical activity? Journal of Cystic Fibrosis 2016;51(Suppl 45):188. [ABSTRACT NO: S20.4] [CFGD REGISTER: PE244a]
-
- NCT01744561. Effects of a partially supervised conditioning program in CF (ACTIVATE-CF) [Effects of a partially supervised conditioning program in CF: an international multi-centre, randomized controlled trial]. clinicaltrials.gov/show/NCT01744561 (first received 5 December 2012). [CENTRAL: 1343886] [CFGD REGISTER: PE244b]
Hommerding 2015 {published data only}
-
- Hommerding PX, Baptista RR, Makarewicz GT, Schindel CS, Donadio MV, Pinto LA, et al. Effects of an educational intervention of physical activity for children and adolescents with cystic fibrosis: a randomized controlled study. Respiratory Care 2015;60(1):81-7. [CFGD REGISTER: PE194b] - PubMed
-
- Marostica PJ, Hommerding P, Makarewicz G, Baptista R, Donadio MV. Efficacy of an educational intervention of physical activity for children and adolescents with cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S36. [ABSTRACT NO: WS16.6] [CFGD REGISTER: PE194a] - PubMed
-
- RBR-3r4h5s. Effect of exercise orientations in the posture and plantar pressure distribution in children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-3r4h5s (first received 9 July 2014). [CFGD REGISTER: PE194d]
-
- Schindel CS, Hommerding PX, Melo DA, Baptista RR, Marostica PJ, Donadio MV. Physical exercise recommendations improve postural changes found in children and adolescents with cystic fibrosis: a randomized controlled trial. Journal of Pediatrics 2015;166(3):710-6. [CFGD REGISTER: PE194c] - PubMed
Klijn 2004 {published data only}
-
- Klijn PH, Oudshoorn A, Ent CK, Net J, Helders PJ. Effects of anaerobic training in children with cystic fibrosis: a randomised controlled study. Chest 2004;125(4):1299-305. [CFGD REGISTER: PE147] - PubMed
Kriemler 2013 {published data only}
-
- Kriemler S, Hebestreit A, Kieser S, Bachmann M, Hebestreit H. Six months of training improves lung function and aerobic performance in CF. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P62. [CFGD REGISTER: PE120a]
-
- Kriemler S, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Effect of supervised training on FEV1 in cystic fibrosis: a randomised controlled trial. Journal of Cystic Fibrosis 2013;12(6):714-20. [CFGD REGISTER: PE120b] - PubMed
Michel 1989 {published data only}
-
- Michel SH, Darbee JC, Pequignot E. Exercise, body composition and strength in cystic fibrosis. Pediatric Pulmonology 1989;4:116. [CFGD REGISTER: PE40]
Moorcroft 2004 {published data only}
-
- Dodd ME, Moorcroft AJ, Webb AK. Improved fitness and decreased symptoms of muscle fatigue for upper and lower body following an individualised unsupervised home exercise training programme in adults with cystic fibrosis. Pediatric Pulmonology 1998;17:347. [CFGD REGISTER: PE97a]
-
- Moorcroft AJ, Dodd ME, Webb AK. Individualised home exercise training in CF – a one-year trial. In: XIIIth International Cystic Fibrosis Congress; 2000 Jun 4-8; Stockholm, Sweden. 2000:153. [CFGD REGISTER: PE97b]
Rovedder 2014 {published data only}
-
- Rovedder PM, Flores J, Ziegler B, Casarotto F, Jaques P, Baretto SS, et al. Exercise programme in patients with cystic fibrosis: a randomized controlled trial. Respiratory Medicine 2014;108(8):1134-40. [CFGD REGISTER: PE214] - PubMed
Santana‐Sosa 2012 {published data only}
-
- Santana-Sosa E, Groeneveld IF, Gonzalez-Saiz L, Lopez-Mojares LM, Villa-Asensi JR, Barrio Gonzalez MI, et al. Intrahospital weight and aerobic training in children with cystic fibrosis: a randomized controlled trial. Medicine and Science in Sports and Exercise 2012;44(1):2-11. [CFGD REGISTER: PE191] - PubMed
Santana‐Sosa 2014 {published data only}
-
- NCT01706445. Combined inspiratory muscle and 'whole muscle' training in children with cystic fibrosis. clinicaltrials.gov/show/NCT01706445 (first received 15 October 2012). [CFGD REGISTER: PE201b]
-
- Santana-Sosa E, Gonzalez-Saiz L, Groeneveld IF, Villa-Asensi JR, Barrio Gomez de Aguero MI, Fleck SJ, et al. Benefits of combining inspiratory muscle with 'whole muscle' training in children with cystic fibrosis: a randomised controlled trial. British Journal of Sports Medicine 2014;48(20):1513-7. [CENTRAL: 874806] [CFGD REGISTER: PE201] [PMID: ] - PubMed
Sawyer 2020 {published data only}
-
- ACTRN12617001271392. Effects of high intensity interval training on exercise capacity in people with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001271392 (first received 4 September 2017). [CFGD REGISTER: PE261c]
-
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Bear N, et al. High-intensity interval training is effective at increasing exercise endurance capacity and is well tolerated by adults with cystic fibrosis. Journal of Clinical Medicine 2020;9(10):3098. [CFGD REGISTER: PE261d] [DOI: 10.3390/jcm9103098] - DOI - PMC - PubMed
-
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. Effects of high intensity interval training on exercise capacity in people with cystic fibrosis: study protocol for a randomised controlled trial. BMC Sports Science, Medicine & Rehabilitation 2018;10(1):19. [CENTRAL: CN-01935836] [CFGD REGISTER: PE261] [EMBASE: 627687202] [PMID: ] - PMC - PubMed
-
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. High intensity interval-based cycle ergometry training is effective at increasing exercise endurance capacity and is well tolerated by adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2020;201(1):C107. [ABSTRACT NO.: A6118] [CFGD REGISTER: PE261b] - PMC - PubMed
-
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. Low-volume high intensity interval training improves exercise endurance capacity and is well tolerated in people with cystic fibrosis: a randomised controlled trial. Respirology (Carlton, Vic.) 2020;25:34. [CFGD REGISTER: PE261e]
Schneiderman‐Walker 2000 {published data only}
-
- Papaioannou M. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. Pediatric Physical Therapy 2001;13(2):94-5. [CENTRAL: 1343880] [CFGD REGISTER: PE58c] [PMID: ] - PubMed
-
- Reisman JJ, Schneiderman-Walker J, Corey M, Wilkes D, Pedder L, Levison H, et al. The role of an organized exercise program in cystic fibrosis – a three year study. Pediatric Pulmonology 1995;Suppl 12:261. [CENTRAL: 291537] [CFGD REGISTER: PE58a]
-
- Schneiderman-Walker J, Pollock SL, Corey M, Wilkes DD, Canny G, Pedder L, et al. A randomised controlled trial of a 3-year home exercise program in cystic fibrosis. Journal of Pediatrics 2000;136(3):304-10. [CFGD REGISTER: PE58b] - PubMed
Selvadurai 2002 {published data only}
-
- Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatric Pulmonology 2002;33(3):194-200. [CFGD REGISTER: PE107b] - PubMed
-
- Selvadurai HC, Asperen PP, Cooper PJ, Mellis CM, Blimkie CJ. A randomised controlled study of in-hospital exercise training programs in children with cystic fibrosis (CF). Pediatric Pulmonology 1999;19:287-8. [CFGD REGISTER: PE107a] - PubMed
Turchetta 1991 {published data only}
-
- Turchetta A, Bella S, Calzolari A, Castro M, Ciuffetti C, Drago F, et al. Effect of controlled physical activity on lung function test of cystic fibrosis children. In: 17th European Cystic Fibrosis Conference; 1991 Jun 18-21; Copenhagen, Denmark. 1991:134. [CFGD REGISTER: PE45]
References to studies excluded from this review
ACTRN12620001237976 {published data only}
-
- ACTRN12620001237976. Virtual models for delivery of exercise training in cystic fibrosis (CF): an evaluation of patient engagement and feasibility. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620001237976 (first registered 18 November 2020). [CFGD REGISTER: PE336]
Alarie 2012 {published data only}
-
- Alarie N, Chan R, Thomas L, Marasco J, Amelie P, Nan W, et al. Cardiorespiratory responses to Nintendo Wii in children with cystic fibrosis: a pilot study. Pediatric Pulmonology 2012;47(S35):367. [ABSTRACT NO: 399] [CFGD REGISTER: PE196]
Albinni 2004 {published data only}
-
- Albinni S, Rath R, Renner S, Eichler I. Additional inspiratory muscle training intensifies the beneficial effects of cycle ergometer training in patients with cystic fibrosis. Journal of Cystic Fibrosis 2004;3(Suppl 1):S63. [CFGD REGISTER: PE148a]
-
- Eichler I, Renner S, Albinni S, Nachbaur E, Rath R. Inspiratory muscle training adds beneficial effects to cycle ergometer training in patients with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):320. [CFGD REGISTER: PE148b]
Almajan‐Guta 2011 {published data only}
-
- Almajan-Guta B, Avram C, Almajan-Guta V, Rusu A, Ciuca I, Cluci O, et al. High motivation for playing sports in cystic fibrosis – what we play is life. Journal of Cystic Fibrosis 2011;10(Suppl 1):S64. [ABSTRACT NO.: 254] [CFGD REGISTER: PE189]
Amelina 2006 {published data only}
-
- Amelina E, Cherniak A, Chikina S, Krasovsky S, Appaeva A. Inspiratory muscle training (IMT) in cystic fibrosis adults. In: European Respiratory Society Annual Congress; 2006 Sep 2-6; Munich, Germany. 2006. [ABSTRACT NO: P4112] [CFGD REGISTER: PE177a]
-
- Cherniak A, Amelina E, Krasovsky S, Nekludova G, Chikina S. The effect of high intensity inspiratory muscle training in adults with cystic fibrosis (CF). European Respiratory Journal 2007;30(Suppl 51):767s. [ABSTRACT NO: E4514] [CENTRAL: 645383] [CFGD REGISTER: PE177b]
Andreasson 1987 {published data only}
-
- Andreasson B, Jonson B, Kornfalt R, Nordmark E, Sandstrom S. Long-term effects of physical exercise on working capacity and pulmonary function in cystic fibrosis. Acta Paediatrica Scandinavica 1987;76(1):70-5. - PubMed
Aquino 2006 {published data only}
-
- Aquino A, Balestri E, Dall Ara S, Lami I, Gobb F, Ambroni M, et al. Efficacy of physical exercise playing a video game for mucus clearance in patients with CF. Journal of Cystic Fibrosis 2006;5(Suppl):S83. [CFGD REGISTER: PE163]
Asher 1982 {published data only}
-
- Asher M, Pardy R, Coates A, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. Australian and New Zealand Journal of Medicine 1983;13:204. [CFGD REGISTER: PE127a] - PubMed
-
- Asher MI, Pardy RL, Coates AL, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. American Review of Respiratory Disease 1982;126(5):855-9. [CFGD REGISTER: PE127b] - PubMed
Balestri 2004 {published data only}
-
- Balestri E, Ambroni M, dall'Ara S, Miano A. Efficacy of physical exercise for mucus clearance in patients with cystic fibrosis (CF). Pediatric Pulmonology 2004;38(S27):316. [CFGD REGISTER: PE150]
Balfour Lynn 1998 {published data only}
-
- Balfour Lynn IM, Prasad SA, Laverty A, Whitehead BF, Dinwiddie R. A step in the right direction: assessing exercise tolerance in cystic fibrosis. Pediatric Pulmonology 1998;25(4):278-84. [CENTRAL: 462732] [CFGD REGISTER: PE69b] - PubMed
-
- Balfour-Lynn IM, Prasad SA, Laverty A, Whitehead BF, Dinwiddie R. Step-test vs 6-minute walk: assessing exercise tolerance in children with cystic fibrosis. Pediatric Pulmonology 1996;13:305. [CFGD REGISTER: PE69a] - PubMed
Barry 2001 {published data only}
-
- Barry S, Dodd J, Jensma M, Gallagher C. Benefits of high intensity strength training in adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A968. [CFGD REGISTER: PE133b]
-
- Barry S, Dodd J, Jensma M, Gallagher C. High intensity strength training improves fitness levels in adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A507. [CFGD REGISTER: PE133a]
Bass 2019 {published data only}
-
- Bass R, Bourke S, Doe S, Diego-Vicente L, Hope E, Smith A, et al. Is "Pactster" an acceptable tool to promote exercise participation in the adult cystic fibrosis community, with and without physiotherapy support? Journal of Cystic Fibrosis 2019;18(Suppl 1):S34. [CENTRAL: CN-01984666] [CFGD REGISTER: PE282]
Bellini 2018 {published data only}
-
- Bellini R, Cazzarolli C. Non-invasive ventilation during exercise in severe cystic fibrosis subjects: a preliminary study. European Respiratory Journal 2018;52(Suppl 62):PA1318. [CFGD REGISTER: OV37]
Bieli 2017 {published data only}
-
- Bieli C, Selina S, Demet I, Andreas J, Alexander M. Respiratory muscle endurance training in cystic fibrosis. European Respiratory Journal 2014;44(Suppl 58):P1972. [CFGD REGISTER: PE237b] [EMBASE: 71851116]
-
- Bieli C, Summermatter S, Boutellier U, Moeller A. Respiratory muscle training improves respiratory muscle endurance but not exercise tolerance in children with cystic fibrosis. Pediatric Pulmonology 2017;52(3):331-6. [CENTRAL: 1262285] [CFGD REGISTER: PE237a] [EMBASE: 614244451] [PMID: ] - PubMed
Bilton 1992 {published data only}
-
- Bilton D, Dodd M, Webb AK. The benefits of exercise combined with physiotherapy in cystic fibrosis. Pediatric Pulmonology 1990;9(Suppl 5):238. [CFGD REGISTER: PE41a]
-
- Bilton D, Dodd ME, Abbot JV, Webb AK. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respiratory Medicine 1992;86(6):507-11. [CFGD REGISTER: PE41b] - PubMed
Bongers 2015 {published data only}
-
- Bongers BC, Werkman MS, Arets HG, Takken T, Hulzebos HJ. A possible alternative exercise test for youths with cystic fibrosis: the steep ramp test. Medicine and Science in Sports and Exercise 2015;47(3):485-92. [CENTRAL: 1096730] [CFGD REGISTER: PE232] [PMID: ] - PubMed
Calik‐Kutukcu 2016 {published data only}
-
- Calik-Kutukcu E, Saglam M, Vardar-Yagli N, Cakmak A, Inal-Ince D, Bozdemir-Ozel C, et al. Listening to motivational music while walking elicits more positive affective response in patients with cystic fibrosis. Complementary Therapies in Clinical Practice 2016;23:52-8. [CENTRAL: 1199953] [CFGD REGISTER: PE234] [PMID: ] - PubMed
Cantin 2005 {published data only}
-
- Cantin AM, Bacon M, Berthiaume Y. Mechanical airway clearance using the Frequencer electro-acoustical transducer in cystic fibrosis. Clinical and Investigative Medicine 2006;29(3):159-65. [CENTRAL: CN-00622988] [CFGD REGISTER: PE159b] [EMBASE: 43927076] - PubMed
-
- Cantin AM, Berthiaume Y. Clearance of airway secretions with the Frequencer in patients with cystic fibrosis. Pediatric Pulmonology 2005;40(S28):322. [ABSTRACT NO.: 379] [CENTRAL: CN-00623756] [CFGD REGISTER: PE159a]
Chang 2015 {published data only}
-
- Chang K, Cotton J, Gashgarian S, Sheppard E, Slack D, Stephenson A, et al. Validation of clinical assessment tools for leg muscle strength and power in adults with cystic fibrosis. Pediatric Pulmonology 2015;50(Suppl 41):368. [ABSTRACT NO: 469] [CENTRAL: 1092168] [CFGD REGISTER: PE223]
Chatham 1997 {published data only}
-
- Chatham K, Ionescu A, Davis C, Baldwin J, Enright S, Shale DJ. Through range computer generated inspiratory muscle training in cystic fibrosis. Pediatric Pulmonology 1997;23(Suppl 14):299. [CFGD REGISTER: PE90]
Combret 2018 {published data only}
-
- Combret Y, Medrinal C, Prieur G, Quesada AR, Le Roux P, Reychler G. Comparative effect of backpack carrying on cystic fibrosis and healthy children: a randomized crossover controlled trial. European Respiratory Journal 2017;50(Suppl 61):PA1341. [ABSTRACT NO.: PA1341] [CENTRAL: CN-01793437] [CFGD REGISTER: PE256b] [DOI: 10.1183/1393003.congress-2017.PA1341 ] [EMBASE: 625792676]
-
- Combret Y, Medrinal C, Prieur G, Robledo Quesada A, Le Roux P, Reychler G. Effect of backpack carrying on forced vital capacity in cystic fibrosis: a randomized crossover-controlled trial. PloS One 2018;13(5):e0196750. [CENTRAL: CN-01621321] [CFGD REGISTER: PE256a] [EMBASE: 622059489] [PMID: ] - PMC - PubMed
Combret 2021 {published data only}
-
- NCT03069625. Sit-to-stand test in cystic fibrosis children and adolescents [Sit-to-stand test use in children and adolescents with cystic fibrosis: correlations with 6-minute walking test, quadriceps and respiratory muscle strength and health related quality of life]. clinicaltrials.gov/show/nct03069625 (first received 3 March 2017). [CENTRAL: CN-01577285]
Cox 2013 {published data only}
-
- Cox NS, Alison JA, Button BM, Wilson JW, Holland AE. Assessing exercise capacity using telehealth: a feasibility study in adults with cystic fibrosis. Respiratory Care 2013;58(2):286-90. [CENTRAL: CN-00968964] [CFGD REGISTER: MH55] [PMID: ] - PubMed
de Jong 1994 {published data only}
-
- De Jong W, Grevink RG, Roorda RJ, Kaptein AA, Schans CP. Effect of a home exercise training program in patients with cystic fibrosis. Chest 1994;105(2):463-8. - PubMed
del Corral Nunez‐Flores 2014 {published data only}
-
- Corral Nunez-Flores T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis. Journal of Cystic Fibrosis 2011;10(Suppl 1):S64. [ABSTRACT NO.: 253] [CFGD REGISTER: PE190a]
-
- Corral Nunez-Flores T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis. Pediatric Pulmonology 2011;46(S34):353. [ABSTRACT NO.: 389] [CFGD REGISTER: PE190b]
-
- Corral T, Percegona J, Seborga M, Rabinovich RA, Vilaro J. Physiological response during activity programs using Wii-based video games in patients with cystic fibrosis. Journal of Cystic Fibrosis 2014;13(6):706-11. [CFGD REGISTER: PE190c] - PubMed
-
- Corral T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis patients. European Respiratory Journal 2011;38(55):878s. [ABSTRACT NO.: P4802] [CENTRAL: 1081933] [CFGD REGISTER: PE190d] [EMBASE: 72122296]
de Marchis 2017 {published data only}
-
- Marchis M, Graziano L, Alatri F, Perelli T, Giacomodonato B, Paris G, et al. Effectiveness of a psychomotor intervention in a group of paediatric patients with cystic fibrosis hospitalized for pulmonary exacerbation: a randomized controlled study. Journal of Cystic Fibrosis 2017;16(Suppl 1):S28-9. [CENTRAL: CN-01461866] [CFGD REGISTER: MH64] [EMBASE: 620749748]
Dwyer 2011 {published data only}
-
- Dwyer T, Alison J, McKeough Z, Daviskas E, Bye P. Exercise aids airway clearance by increasing respiratory flow rates and decreasing mucus viscoelasticity in CF. Pediatric Pulmonology 2008;43(Suppl 31):386. [ABSTRACT NO.: 513] [CFGD REGISTER: PE188a]
-
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139(4):870-7. [CENTRAL: CN-00785593] [CFGD REGISTER: PE188b] [PMID: ] - PubMed
Dwyer 2017 {published data only}
-
- Dwyer TJ, Zainuldin R, Daviskas E, Bye PT, Alison JA. Effects of treadmill exercise versus Flutter(R) on respiratory flow and sputum properties in adults with cystic fibrosis: a randomised, controlled, cross-over trial. BMC Pulmonary Medicine 2017;17(1):14. [CFGD REGISTER: PE239] [PMID: ] - PMC - PubMed
Dwyer 2019 {published data only}
-
- ACTRN12608000287336. Does exercise enhance mucociliary clearance in adults with cystic fibrosis? www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000287336 (first received 4 June 2008). [CFGD REGISTER: PE274b]
-
- Dwyer TJ, Daviskas E, Zainuldin R, Verschuer J, Eberl S, Bye PT, et al. Effects of exercise and airway clearance (positive expiratory pressure) on mucus clearance in cystic fibrosis: a randomised crossover trial. European Respiratory Journal 2019;53(4):pii: 1801793. [CENTRAL: CN-01938004] [CFGD REGISTER: PE274a] [DOI: 10.1183/13993003.01793-2018] [EMBASE: 627483418] [PMID: ] - DOI - PubMed
Edlund 1986 {published data only}
-
- Adams TD, Edland LL, French RW, Herbst JJ, Ruttenberg HD, Ruhling RO. Effects of a swimming program on children with cystic fibrosis. International Journal of Sports Medicine 1984;5:156. [CFGD REGISTER: PE154a] - PubMed
-
- Edlund LD, French RW, Herbst JJ, Ruttenburg HD, Ruhling RO, Adams TD. Effects of a swimming program on children with cystic fibrosis. American Journal of Diseases of Children 1986;140(1):80-3. [CFGD REGISTER: PE154b] - PubMed
Falk 1988 {published data only}
-
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise. Effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. In: 10th International Cystic Fibrosis Congress; 1988 Mar 5-10; Sydney, Australia. 1988:35. [CENTRAL: CN-00208301]
-
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise – effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. Excerpta Medica, Asia Pacific Congress Series 1988;74:35. [CFGD REGISTER: PE36]
Giacomodonato 2015 {published data only}
-
- Giacomodonato B, Graziano L, Curzi M, Perelli T, De Sanctis S, Varchetta M, et al. Respiratory muscle endurance training with normocapnic hyperpnea in patients with cystic fibrosis. A randomized controlled study. Journal of Cystic Fibrosis 2015;14(Suppl 1):S41. [ABSTRACT NO: WS21.10] [CENTRAL: 1077187] [CFGD REGISTER: PE217]
Gruber 1998 {published data only}
-
- Gruber W, Kiosz D, Braumann KM, Kolbel R. Exercise and cystic fibrosis. A comparison of training programs with different frequency of training. International Journal of Sports Medicine 1998;19(Suppl):S16. [CENTRAL: CN-00493762] [CFGD REGISTER: PE285]
Gruet 2012 {published data only}
-
- Gruet M, Mely L, Brisswalter J, Vallier J-M. Neuromuscular electrical stimulation in cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S109. [ABSTRACT NO.: 208] [CFGD REGISTER: PE195]
Happ 2013 {published data only}
-
- NCT00609050. Exercise training study for patients with cystic fibrosis [Self-regulated exercise in CF: a randomized trial]. clinicaltrials.gov/ct2/show/NCT00609050 (first received 31 January 2008). [CFGD REGISTER: PE307a]
Haynes 2016 {published data only}
-
- Haynes F, Maddison L, Millward S, Hughes T, Dewar J. Evaluation of the incremental step test with adult CF patients. Journal of Cystic Fibrosis 2016;15(Suppl):S88. [ABSTRACT NO: 146] [CENTRAL: 1171474] [CFGD REGISTER: PE233]
Heijerman 1992 {published data only}
-
- Heijerman HG, Bakker W, Sterk PJ, Dijkman JH. Long-term effects of exercise training and hyperalimentation in adult cystic fibrosis patients with severe pulmonary dysfunction. International Journal of Rehabilitation Research 1992;15(3):252-7. - PubMed
Housinger 2015 {published data only}
-
- Housinger E, Sullivan M, Ruiz FE. Effects of an interdisciplinary motivational incentive-based walking program in pediatric pulmonary patients during hospitalization. Pediatric Pulmonology 2015;50(Suppl 41):369. [ABSTRACT NO: 471] [CENTRAL: 1092214] [CFGD REGISTER: PE222] [EMBASE: 72081750]
Hütler 2002 {published data only}
-
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, Böning D, et al. Effect of growth hormone on exercise tolerance in children with cystic fibrosis. Medicine and Science in Sports and Exercise 2002;34(4):567-72. [CFGD REGISTER: GN125a] - PubMed
-
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, Boning D. Growth hormone enhances peak performance in cystic fibrosis. International Journal of Sports Medicine 1998;19(Suppl):S17. [CENTRAL: CN-00494036] [CFGD REGISTER: GN125b]
IRCT20161024030474N4 {published data only}
-
- IRCT20161024030474N4. Effect of pulmonary rehabilitation on quality of life in children with cystic fibrosis and their mother's caregiver burden. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20161024030474N4 (first received 7 June 2020). [CFGD REGISTER: PE326]
Irons 2012 {published data only}
-
- ACTRN12609000471280. Let's sing out!: the effect of singing on quality of life and lung function of children and adolescents with cystic fibrosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ID=83944 (first received 16 June 2009). [CFGD REGISTER: PE185a]
-
- Irons JY, Kenny DT, McElrea M, Chang AB. Singing therapy for young people with cystic fibrosis: a randomized controlled pilot study. Music and Medicine 2012;4(3):136-45. [CENTRAL: 861294] [CFGD REGISTER: PE185b]
Johnston 2004 {published data only}
-
- Johnston K, Jenkins, Roberts C, Stick S. Improved attitude to exercise in overweight children with lung conditions after an exercise intervention. Respirology 2004;9(2 Suppl):A60. [CENTRAL: 475664] [CFGD REGISTER: PE231]
Kaak 2011 {published data only}
-
- Kaak I, Helbig I, Ankermann T. Didgeridoo playing as an adjunctive therapy to conventional physiotherapy for patients with cystic fibrosis. Zeitschrift fur Physiotherapeuten 2011;63(2):6-14. [CFGD REGISTER: PE292]
Kaltsakas 2021 {published data only}
-
- Kaltsakas G, Anastasopoulos N, Chynkiamis N, Zeliou P, Karapatoucha V, Kotsifas K, et al. Effect of high intensity interval exercise rehabilitation in cystic fibrosis. European Respiratory Journal 2017;50(Suppl 61):OA310. [CENTRAL: CN-01788028] [CFGD REGISTER: PE278b] [DOI: 10.1183/1393003.congress-2017] [EMBASE: 625788657] - DOI
-
- Kaltsakas G, Anastasopoulos N, Chynkiamis N, Zeliou P, Karapatoucha V, Kotsifas K, et al. Functional capacity, peripheral muscle strength, and quality of life following interval versus continuous rehabilitative exercise training in cystic fibrosis. Thorax 2017;72(Suppl 3):A51. [CENTRAL: CN-01437830] [CFGD REGISTER: PE278a] [EMBASE: 619738976]
Kriemler 2016 {published data only}
-
- Kriemler S, Radtke T, Christen G, Kerstan-Huber M, Hebestreit H. Short-term effect of different physical exercises and physiotherapy combinations on sputum expectoration, oxygen saturation, and lung function in young patients with cystic fibrosis. Lung 2016;194(4):659-64. [CFGD REGISTER: PE219b] [PMID: ] - PubMed
-
- Radtke T, Christen G, Kerstan Huber M, Hebestreit H, Kriemler S. Short-term effect of different physical exercise–physiotherapy combinations on sputum production, oxygen saturation and lung function in young patients with cystic fibrosis. Journal of Cystic Fibrosis 2015;14(Suppl 1):S27. [ABSTRACT NO: WS14.2] [CENTRAL: 1081483] [CFGD REGISTER: PE219a] [EMBASE: 71951559] - PubMed
Kuys 2011 {published data only}
-
- Hall K, Peasey M, Wood M, Cobb R, Bell S, Kuys S. The effects of Nintendo-Wii exercise training in adults with cystic fibrosis. Physiotherapy (United Kingdom) 2011;97(Suppl 1):eS648. [CENTRAL: 1089274] [CFGD REGISTER: PE184c] [EMBASE: 71883056]
-
- Hall K, Peasey M, Wood M, Cobb R, Bell SC, Kuys S. The effects of Nintendo-Wii® exercise training in adults with cystic fibrosis. Respirology (Carlton, Vic.) 2011;16(Suppl 1):P56. [ABSTRACT NO: TP 077] [CFGD REGISTER: PE184d]
-
- Hall K, Peasey M, Wood M, Cobb R, Bell SC, Kuys S. The effects of Nintendo Wii exercise training in adults with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(Suppl 1):A275. [CFGD REGISTER: PE184a]
-
- Kuys SS, Hall K, Peasey M, Wood M, Cobb R, Bell SC. Gaming console exercise and cycle or treadmill exercise provide similar cardiovascular demand in adults with cystic fibrosis: a randomised cross-over trial. Journal of Physiotherapy 2011;57(1):35-40. [CFGD REGISTER: PE184b] - PubMed
Lang 2019 {published data only}
-
- ACTRN12617001035314. CyFiT Telerehabilitation: technology based physiotherapy for peer driven participation in therapy, and quality of life. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001035314 (first received 17 July 2017). [CFGD REGISTER: PE305a]
Lannefors 1992 {published data only}
-
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis (CF) – a comparison between postural drainage, PEP-mask and physical exercise. In: 11th International Cystic Fibrosis Congress; 1992 Aug 22-27; Dubin, Ireland. 1992:AHP31. [CFGD REGISTER: PE46a] - PubMed
-
- Lannefors L, Wollmerr P. Mucus clearance with three chest physiotherapy regimes in CF: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748-53. [CFGD REGISTER: PE46b] - PubMed
Lima 2014 {published data only}
-
- Lima C, De Andrade AD, Rattes C, Campos S, Brandao D, Aliverti A, et al. Effect of noninvasive ventilation on functional exercise capacity, lung function and compartmental chest wall volume in children with cystic fibrosis. European Respiratory Journal 2013;42:1075s. [ABSTRACT NO: P5065] [CENTRAL: 1099891] [CFGD REGISTER: PE216b] [EMBASE: 71843506]
-
- Lima CA, De Andrade AD, Campos SL, Brandao DC, Fregonezi G, Mourato IP, et al. Effects of noninvasive ventilation on treadmill 6-min walking distance and regional chest wall volumes in cystic fibrosis: randomized controlled trial. Respiratory Medicine 2014;108(10):1460-8. [CFGD REGISTER: PE216a] - PubMed
-
- NCT01987271. Effects of noninvasive ventilation on functional capacity of patients with cystic fibrosis [Effects of noninvasive ventilation during the treadmill walking test on cardiorespiratory system, walk distance, and thoracoabdominal kinematics of patients with cystic fibrosis: clinical randomized controlled trial]. clinicaltrials.gov/show/NCT01987271 (first received 19 November 2013). [CENTRAL: CN-01479093] [CFGD REGISTER: PE216c]
Lowman 2012 {published data only}
-
- Lowman JD, Britton LJ, Lee LS, Phillips AL, Hoover WC. Comprehensive exercise training during hospitalization for an acute CF exacerbation: a randomized controlled trial. Journal of Cystic Fibrosis 2012;11(Suppl 1):S23. [ABSTRACT NO.: WS10.5] [CFGD REGISTER: PE192]
Macleod 2008 {published data only}
-
- Horsley A, Ridley S, Beattie C, Macleod K, Greening A, Innes JA. Changes in lung gas mixing after physiotherapy in adults with cystic fibrosis. European Respiratory Journal 2007;30(Suppl 51):223s. [CENTRAL: CN-00645484] [CFGD REGISTER: PE252a]
-
- Macleod K, Dhouieb E, Riding K, Horsley A, Bell N, Alistair Innes J, et al. Acute changes in ventilation heterogeneity following routine physiotherapy in children with cystic fibrosis. In: European Respiratory Society Annual Congress; 2008 Oct 4-8; Berlin, Germany. 2008:P3903. [CENTRAL: CN-00679234] [CFGD REGISTER: PE252b]
Mandrusiak 2011 {published data only}
-
- ACTRN12607000612415. Effect of an exercise program on function, activity and participation of young people with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12607000612415 (first received 28 November 2007). [CFGD REGISTER: PE229b]
-
- Mandrusiak A, MacDonald J, Paratz J, Wilson C, Watter P. A novel exercise program for young people with cystic fibrosis: moving physiotherapy forward through targeted design. Physiotherapy 2011;97:eS1550. [CENTRAL: 1076180] [CFGD REGISTER: PE229] [EMBASE: 71884245] [POSTER NO.: SI-PO-210-22-Thu]
Martinez Rodriguez 2017 {published data only}
-
- Martinez Rodriguez ME, Suarez Cortina L, Maiz Carro L, Ruiz-De-Valbuena M, Jimenez Cosmes L. A pulmonary rehabilitation program to increase adherence to airway clearance techniques for children and adults with cystic fibrosis. Journal of Cystic Fibrosis 2017;16(Suppl 1):S58. [CENTRAL: CN-01461867] [CFGD REGISTER: PE248] [EMBASE: 620749723]
Montero‐Ruiz 2020 {published data only}ISRCTN11161411
-
- Frias JP, Montero-Ruiz A, Galvez LA, Perez-Ruiz E, Huelamo MP, Martin-Montanez E. A music therapy intervention as an adjunct to chest physiotherapy in children with cystic fibrosis. European Respiratory Journal 2018;52(Suppl 62):PA4626. [CFGD REGISTER: PE293b]
Moola 2017 {published data only}
-
- Moola FJ, Garcia E, Huynh E, Henry L, Penfound S, Consunji-Araneta R, et al. Physical activity counselling for children with cystic fibrosis. Respiratory Care 2017;62(11):1466-73. [CENTRAL: CN-01411945] [CFGD REGISTER: PE247] [PMID: ] - PubMed
NCT00129350 {published data only}
-
- NCT00129350. Assessment of heart and heart-lung transplant patient outcomes following pulmonary rehabilitation. clinicaltrials.gov/show/NCT00129350 (first received 11 August 2005). [CFGD REGISTER: CO92]
NCT00792194 {published data only}
-
- NCT00792194. Improvement of aerobic capacity in cystic fibrosis patients with a one-year home training period. clinicaltrials.gov/ct2/show/NCT00792194 (first received 14 November 2008).
NCT01759342 {published data only}
-
- NCT01759342. Comprehensive exercise training program during hospitalization for an acute CF exacerbation. clinicaltrials.gov/show/NCT01759342 (first received 3 January 2013). [CFGD REGISTER: PE315]
NCT02199340 {published data only}
-
- NCT02199340. The iStep Study: development and validation of an incremental exercise step test for children with cystic fibrosis. clinicaltrials.gov/show/NCT02199340 (first received 24 July 2014). [CFGD REGISTER: PE313]
NCT02277860 {published data only}
-
- NCT02277860. Gaming console home-based exercise for adults with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02277860 (first received 27 October 2014).
NCT02715921 {published data only}
-
- NCT02715921. Impact of telerehabilitation training on pediatric cystic fibrosis patients: an exploratory study. clinicaltrials.gov/ct2/show/NCT02715921 (first received 12 January 2016).
NCT02821130 {published data only}
-
- NCT02821130. Orkambi exercise study (Orkambi) [Effects of Orkambi on exertional dyspnea, exercise performance, and ventilatory responses in adults with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT02821130 (first received 6 June 2016).
NCT02875366 {published data only}
-
- NCT02875366. A study of the effects of lumacaftor/ivacaftor on exercise tolerance in subjects with cystic fibrosis, homozygous for the F508del-CFTR mutation [A phase 4, randomized, double-blind, placebo-controlled, parallel-design study of the effect of lumacaftor/ivacaftor combination therapy on exercise tolerance in subjects aged 12 years and older with cystic fibrosis, homozygous for the F508del-CFTR mutation]. clinicaltrials.gov/ct2/show/NCT02875366 (first received 15 August 2016).
NCT03117764 {published data only}
-
- NCT03117764. Evaluation of the impact of intravenous antibiotics on muscular strength in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03117764 (first received 31 March 2017).
NCT03420209 {published data only}
-
- NCT03420209. The effect of proprioceptive neuromuscular facilitation (PNF) technique for children with chronic pulmonary diseases. clinicaltrials.gov/show/nct03420209 (first received 05 February 2018). [CFGD REGISTER: PE339]
NCT04888767 {published data only}
-
- NCT04888767. Safety and feasibility of high-intensity interval training program in CF patients. clinicaltrials.gov/show/NCT04888767 (first received 17 May 2021). [CFGD REGISTER: PE338]
NTR2092 {published data only}
-
- NTR2092. Inspiratory muscle training prior to peripheral muscle training in children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR2092 (first received 4 November 2009). [CFGD REGISTER: PE314]
Oliveira 2010 {published data only}
-
- Oliveira AC, Jesus TA, Mesquita-Ferrari RA, Oliveira JC, Oliveira LV, Sampaio LM. Aerobic training and quality of life in cystic fibrosis. European Respiratory Journal 2010;36(Suppl 54):P2651. [CFGD REGISTER: PE245]
Orenstein 1981 {published data only}
-
- Orenstein DM, Franklin BA, Doershuk CF, Hellerstein HK, Germann KJ, Horowitz JG, et al. Exercise conditioning and cardiopulmonary fitness in CF. The effects of a three-month supervised running program. Chest 1981;80(4):392-8. [CFGD REGISTER: PE92] - PubMed
Orenstein 2004 {published data only}
-
- Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter CR, Kelsey S, et al. Strength vs aerobic training in children with cystic fibrosis: a randomised controlled trial. Chest 2004;126(4):1204-14. [CFGD REGISTER: PE165] - PubMed
Ozaydin 2010 {published data only}
-
- Ozaydin Z, Savci S, Saglam M, Arikan H, Inal-Ince D, Vardar-Yagli N, et al. Effects of inspiratory muscle training on functional capacity and muscle strength in patients with mild cystic fibrosis. In: European Respiratory Society Annual Congress; 2010 Sep 18-22; Barcelona, Spain. 2010. [ABSTRACT NO: 5134] [CENTRAL: 777297] [CFGD REGISTER: PE235]
Patterson 2004 {published data only}
-
- Patterson JE, Bradley JM. Inspiratory muscle training in adult patients with cystic fibrosis: a randomised controlled trial to evaluate the efficacy of the test of incremental respiratory endurance (TIRE). Thorax 2004;59(Suppl II):ii13. [ABSTRACT NO: S36] [CENTRAL: 518434] [CFGD REGISTER: PE236]
Petrovic 2013 {published data only}
-
- Petrovic M, Kaluza I, Pohl W. Effects of individualised aerobic exercise training in adults with cystic fibrosis: a 4 year controlled trial. Journal of Cystic Fibrosis 2013;12(Suppl 1):S28. [ABSTRACT NO.: WS14.4] [CENTRAL: 875002] [CFGD REGISTER: PE204]
Phillips 2008 {published data only}
-
- Phillips AL, Lee L, Britton LJ, Hoover W, Lowman JD. Efficacy of a standardised exercise protocol in inpatient care of patients with cystic fibrosis. Pediatric Pulmonology 2008;43(Suppl 31):385. [ABSTRACT NO: 510] [CFGD REGISTER: PE182]
Pryor 1979 {published data only}
-
- Hodson ME, Batten JC, Pryor JA, Webber BA. Evaluation of the forced expiration technique as an adjunct to postural drainage in the treatment of cystic fibrosis. In: 9th Meeting European Working Group for Cystic Fibrosis; 1979 Jun 12-13; Noordwijkerhout, Netherlands. 1979:57. [CFGD REGISTER: PE78b]
-
- Pryor JA, Webber BA. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979;65(10):304-7. [CENTRAL: CN-00208127] [CFGD REGISTER: PE78c] - PubMed
Radtke 2018b {published data only}
-
- NCT02750722. Exercise and oscillatory positive expiratory pressure therapy in cystic fibrosis [Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusion capacity in cystic fibrosis: a randomized crossover trial]. clinicaltrials.gov/show/NCT02750722 (first received 25 April 2016). [CENTRAL: CN-01557680] [CFGD REGISTER: PE255d]
-
- Radtke T, Boeni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. European Respiratory Journal 2018;52(Suppl 62):PA3418. [CENTRAL: CN-01915241] [CFGD REGISTER: PE255e] [EMBASE: 626624799] - PMC - PubMed
-
- Radtke T, Boni L, Bohnacker P, Fischer P, Benden C, Dressel H. The many ways sputum flows – dealing with high within-subject variability in cystic fibrosis sputum rheology. Respiratory Physiology & Neurobiology 2018;254:36-9. [CENTRAL: CN-01607707] [CFGD REGISTER: PE255c] [EMBASE: 2000702039] [PMID: ] - PubMed
-
- Radtke T, Boni L, Bohnacker P, Maggi-Beba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. BMC Pulmonary Medicine 2018;18(1):99. [CENTRAL: CN-01611277] [CFGD REGISTER: PE255a] [EMBASE: 622563058] [PMID: ] - PMC - PubMed
-
- Radtke T, Boni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomised, controlled, crossover trial. Journal of Cystic Fibrosis 2018;17(Suppl 3):S15. [CENTRAL: CN-01746901] [CFGD REGISTER: PE255b] [EMBASE: 622931153] - PMC - PubMed
Rand 2012 {published data only}
-
- Rand S, Hill L, Prasad SA, Main E. Development of an incremental field exercise test for children with cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S36. [ABSTRACT NO.: WS16.7] [CFGD REGISTER: PE193]
RBR‐34677v {published data only}
-
- RBR-34677v. Use of videogames as physical training for children and adolescents with cystic fibrosis during hospitalization. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-34677v (first received 9 June 2015). [CFGD REGISTER: PE316]
RBR‐5g9f6w {published data only}
-
- RBR-5g9f6w. Rehabilitation of children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-5g9f6w (first received 17 June 2020). [CFGD REGISTER: PE324]
Reix 2012 {published data only}
-
- NCT01509235. Self drainage in pediatric cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT01509235 (first received 12 January 2012). [CFGD REGISTER: PE183c]
-
- Reix P, Aubert F, Kassai B, Bige V, Bellon G. Better satisfaction of cystic fibrosis paediatric patients with autogenic drainage associated to exercise compared to conventional chest physiotherapy. Journal of Cystic Fibrosis 2009;8(Suppl 2):S73. [ABSTRACT NO.: 293] [CFGD REGISTER: PE183a]
-
- Reix P, Aubert F, Werck-Gallois MC, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2012;58(4):241-7. [CENTRAL: 841658] [CFGD REGISTER: PE183b] [PMID: ] - PubMed
Reuveny 2020 {published data only}
-
- ISRCTN13864650. Using oxygen to improve exercise performance in patients with cystic fibrosis. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN13864650 (first received 16 July 2019). [CFGD REGISTER: PE294b]
-
- Reuveny R, Dimenna FJ, Gunaratnam C, Arad AD, McElvaney GN, Susta D, et al. High-intensity interval training accelerates oxygen uptake kinetics and improves exercise tolerance for individuals with cystic fibrosis. BMC Sports Science, Medicine and Rehabilitation 2020;12(1):13. [CFGD REGISTER: PE294a] [DOI: 10.1186/s13102-020-0159-z] - DOI - PMC - PubMed
Ruddy 2015 {published data only}
-
- NCT01325766. Study of yoga as a therapy for cystic fibrosis (CF) patients. clinicaltrials.gov/show/NCT01325766 (first received 30 March 2011). [CFGD REGISTER: PE337a]
Salh 1989 {published data only}
Salonini 2015 {published data only}
-
- Salonini E, Gambazza S, Meneghelli I, Tridello G, Sanguanini M, Cazzarolli C, et al. Active video game playing in children and adolescents with cystic fibrosis: exercise or just fun? Respiratory Care 2015;60(8):1172-9. [CENTRAL: 1161387] [CFGD REGISTER: PE207b] [EMBASE: 2015465751] - PubMed
-
- Salonini E, Gambazza S, Tridello G, Sanguanini M, Cazzarolli C. Kinect active video game in cystic fibrosis: exercise or fun? Journal of Cystic Fibrosis 2013;12(Suppl 1):S108. [ABSTRACT NO.: 233] [CENTRAL: 875196] [CFGD REGISTER: PE207a]
Shaw 2016 {published data only}
Spoletini 2020 {published data only}
-
- NCT03965832. HFNT during exercise in CF [A pilot study to evaluate the feasibility of using high-flow nasal therapy during exercise in patients with cystic fibrosis and severe lung disease]. clinicaltrials.gov/show/NCT03965832 (first received 29 May 2019). [CFGD REGISTER: PE308a]
-
- Spoletini G, Watson R, Pollard K, Lim WY, Etherington C, Clifton I, et al. Nasal high-flow therapy (NHFT) during exercise in patients with cystic fibrosis (CF): a randomized crossover trial. European Respiratory Journal 2020;56(Suppl 64):365. [CFGD REGISTER: PE308b]
Stanghelle 1998 {published data only}
-
- Stanghelle JK, Hjeltnes N, Bangstad HJ, Michalsen H. Effect of daily short bouts of trampoline exercise during 8 weeks on the pulmonary function and the maximal oxygen uptake of children with cystic fibrosis. International Journal of Sports Medicine 1988;9(Suppl 1):32-6. [CENTRAL: 568629] [CFGD REGISTER: PE155] [PMID: ] - PubMed
Tuzin 1998 {published data only}
-
- Tuzin BJ, Mulvihill MM, Kilbourn KM, Bertran DA, Buono M, Hovell MF, et al. Increasing physical activity of children with cystic fibrosis: a home based family intervention. Pediatric Exercise Science 1998;10(1):57-68.
Vallier 2016 {published data only}
-
- Vallier JM, Rouissi M, Mely L, Gruet M. Physiological responses of the modified shuttle test in adults with cystic fibrosis. Journal of Cardiopulmonary Rehabilitation and Prevention 2016;36(4):288-92. [CENTRAL: 1343882] [CFGD REGISTER: PE241] [PMID: ] - PubMed
Vivodtzev 2013 {published data only}
-
- NCT00391703. Assessment of quadriceps muscle electrostimulation used in patients suffering from cystic fibrosis. clinicaltrials.gov/show/NCT00391703 (first received 24 October 2006). [CFGD REGISTER: PE210c]
-
- Vivodtzev I, Decorte N, Wuyam B, Gonnet N, Durieu I, Levy P, et al. Benefits of neuromuscular electrical stimulation prior to endurance training in patients with cystic fibrosis and severe pulmonary dysfunction. Chest 2013;143(2):485-93. [CENTRAL: 980643] [CFGD REGISTER: PE201b] - PubMed
-
- Vivodtzev I, Decorte N, Wuyam B, Gonnet N, Durieu I, Levy P, et al. Benefits of neuromuscular electrical stimulation prior to endurance training in patients with cystic fibrosis and severe pulmonary dysfunction. Chest 2013;143(2):485-93. [CENTRAL: 906021] [CFGD REGISTER: PE201a] [EMBASE: 22911373] [PMID: ] - PubMed
Ward 2018 {published data only}
-
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis: clinical outcomes. Respirology 2018;23(Suppl 1):140. [CENTRAL: CN-01607201] [CFGD REGISTER: PE257a] [EMBASE: 622091390] - PubMed
-
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. Respiratory Medicine 2018;142:23-8. [CENTRAL: CN-01616954] [CFGD REGISTER: PE257b] [EMBASE: 2000968825] - PubMed
Wheatley 2015 {published data only}
-
- Wheatley CM, Baker SE, Morgan MA, Martinez MG, Liu B, Rowe SM, et al. Moderate intensity exercise mediates comparable increases in exhaled chloride as albuterol in individuals with cystic fibrosis. Respiratory Medicine 2015;109(8):1001-11. [CENTRAL: 1107333] [CFGD REGISTER: PE225b] [EMBASE: 2015118578] - PMC - PubMed
White 1997 {published data only}
-
- Stiller K. Are thoracic expansion exercises necessary during the active cycle of breathing techniques for adult cystic fibrosis patients? Israel Journal of Medical Sciences 1996;32(Suppl):S275. [CFGD REGISTER: PE61a]
-
- White D, Stiller K, Willson K. The role of thoracic expansion exercises during the active cycle of breathing techniques. Physiotherapy Theory and Practice 1997;13(2):155-62. [CENTRAL: CN-00448712] [CFGD REGISTER: PE61b]
Young 2019 {published data only}
-
- Young R, Wilson P, Underhill B, Shafi N, Watson D. Comparison of the incremental Shuttle Walk Test for adult subjects with cystic fibrosis in two formats: hallway versus treadmill. Journal of Cystic Fibrosis 2019;18(Suppl 1):S161. [CENTRAL: CN-01984664] [CFGD REGISTER: PE280]
Zeren 2019 {published data only}
-
- NCT03375684. Effects of inspiratory muscle training on postural stability, balance, pulmonary function and functional capacity in children with cystic fibrosis. clinicaltrials.gov/show/NCT03375684 (first received 18 December 2017). [CFGD REGISTER: PE275c]
-
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Respiratory Medicine 2019;148:24-30. [CFGD REGISTER: PE275a] - PubMed
-
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Turkish Thoracic Journal 2019;20:S106. [CFGD REGISTER: PE275b] - PubMed
References to studies awaiting assessment
Bishay 2017 {published data only}
-
- Bishay LC, Gould A, Prushingskaya O, Hill K, Greenberg J, Ng J, et al. Baseline submaximal exercise tolerance as an outcome measure in a cystic fibrosis exercise study. American Journal of Respiratory and Critical Care Medicine 2017;195:C72. [ABSTRACT NO.: A6259] [CENTRAL: CN-01408842] [CFGD REGISTER: MH60c] [EMBASE: 617709675]
-
- Bishay LC, Gould A, Prushinskaya OV, Hill K, Greenberg J, Sawicki GS, et al. Acceptability of a fitness tracker for patients with CF: a qualitative analysis within a randomized trial. Pediatric Pulmonology 2017;52(S47):490. [ABSTRACT NO.: 707] [CENTRAL: CN-01430881] [CFGD REGISTER: MH60a] [EMBASE: 619069615]
-
- Bishay LC, Nelson E, Williams K, Greenberg J, Gould A, Bailey I, et al. Effect of a wearable fitness tracker on exercise tolerance for adults with cystic fibrosis: a pilot randomized clinical trial. Pediatric Pulmonology 2018;53(S2):345. [ABSTRACT NO.: 516] [CENTRAL: CN-01738935] [CFGD REGISTER: MH60b] [EMBASE: 624049022]
-
- NCT02700243. Increase tolerance for exercise and raise activity through connectedness trial (INTERACT) [Pilot RCT study using a Fitbit device to improve exercise tolerance in 80 patients with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT02700243 (first received 21 February 2016). [CFGD REGISTER: MH60d]
Cox 2019 {published data only}
-
- ACTRN12617001009303. Action: PACT. Be Active. Online. A trial to promote physical activity in young people with cystic fibrosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617001009303 (first received 13 July 2017). [CFGD REGISTER: PE287b]
IRCT20190407043190N1 {published data only}
-
- IRCT20190407043190N1. Effect of the physical activity plan on the quality of life in children with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190407043190N1 (first received 4 December 2019). [CFGD REGISTER: PE320]
NCT03100214 {published data only}
-
- NCT03100214. Effects of an early rehabilitation program during hospitalization in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03100214 (first received 31 January 2017). [CFGD REGISTER: PE317]
NCT04293926 {published data only}
-
- NCT04293926. Heart rate variability in children and adolescents with cystic fibrosis. clinicaltrials.gov/show/NCT04293926 (first received 3 March 2020). [CFGD REGISTER: PE321]
Powers 2016 {published data only}
-
- NCT03109912. Do More, B'More, Live Fit [Do More, B'More, Live Fit: an outpatient fitness-training pilot program designed to optimize the habit of exercise in adolescents and young adults with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT03109912 (first received 12 April 2017). [CENTRAL: CN-01380971] [CFGD REGISTER: PE246a]
-
- Powers KE, Herzog T, Loosen H, Berg K, Weir B, Riekert KA, et al. Step It Up: higher step count is significantly correlated with better exercise capacity in individuals with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2017;195(Abstract Issue):A6135. [CENTRAL: CN-01409315] [CFGD REGISTER: PE246d] [EMBASE: 617704603]
-
- Powers KE, Herzog TL, Loosen H, Berg K, Weir B, Riekert KA, et al. Self-reported physical activity does not correlate with ventilation inhomogeneity. Pediatric Pulmonology 2016;51(S45):370. [CENTRAL: CN-01212541] [CFGD REGISTER: PE246c] [EMBASE: 612359219]
-
- Powers KE, Loosen H, Berg K, Herzog TL, Weir B, Riekert KA, et al. Ventilation inhomogeneity and lung function are associated with exercise capacity. Pediatric Pulmonology 2016;51(S45):369-70. [CENTRAL: CN-01212542] [CFGD REGISTER: PE246b] [EMBASE: 612359207]
References to ongoing studies
Curran 2020 {published data only}
-
- Curran M, Tierney AC, Collins L, Kennedy L, McDonnell C, Jurascheck AJ, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis: study protocol for a pilot randomised trial using wearable technology, goal setting and text message feedback. HRB Open Research 2020;3:21. [CFGD REGISTER: PE309b] [DOI: 10.12688/hrbopenres.13025.3] [CLINICALTRIALS.GOV: NCT03672058] - DOI - PMC - PubMed
-
- NCT03672058. Steps Ahead: optimising physical activity and health in adults with cystic fibrosis. clinicaltrials.gov/show/NCT03672058 (first received 14 September 2018). [CFGD REGISTER: PE309a]
ISRCTN92573472 {published data only}
-
- ISRCTN92573472. The evaluation of a 12-week partially supervised, self-regulated exercise intervention in patients with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN92573472 (first received 24 February 2020). [CFGD REGISTER: PE323]
Monteiro 2019 {published data only}
-
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 1 SPIRIT 2013 checklist. [CFGD REGISTER: PE288c] - PMC - PubMed
-
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 2 booklet. [CFGD REGISTER: PE288d] - PMC - PubMed
-
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 3 home data recording diary. [CFGD REGISTER: PE288e] - PMC - PubMed
-
- NCT03653949. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03653949 (first received 31 August 2018). [CFGD REGISTER: PE288b]
NCT03273959 {published data only}
-
- NCT03273959. Program of exercises during the hospitalization of children and adolescents with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03273959 (first received 6 September 2017). [CFGD: REGISTER: PI318]
NCT03970369 {published data only}
-
- NCT03970369. Motivated to move: a study to determine the feasibility of self-monitoring physical activity in youth. clinicaltrials.gov/ct2/show/NCT03970369 (first received 31 May 2019).
NCT04249999 {published data only}
-
- NCT04249999. ActivOnline: physical activity in cystic fibrosis trial UK (ActiOnPACTUK). clinicaltrials.gov/show/NCT04249999 (first received 31 January 2020). [CFGD REGISTER: PE303]
NCT04543929 {published data only}
-
- NCT04543929. Effects of innovative aerobic exercise training in cystic fibrosis. clinicaltrials.gov/show/NCT04543929 (first received 10 September 2020). [CFGD REGISTER: PE322]
NCT04683809 {published data only}
-
- NCT04683809. Effects of a telerehabilitation approach in children with cystic fibrosis [The effects of telerehabilitation on quality of life, anxiety and depression levels in children with cystic fibrosis and their caregivers]. clinicaltrials.gov/ct2/show/NCT04683809 (first received 24 December 2020).
NCT04742049 {published data only}
-
- NCT04742049. The effects of telerehabilitation on muscle function, physical activity and sleep in cystic fibrosis during pandemic. clinicaltrials.gov/show/NCT04742049 (first received 5 February 2021). [CFGD REGISTER: PE325]
NCT05147285 {published data only}
-
- NCT05147285. The effect of telerehabilitation on functional capacity, oxidative stress and respiratory parameters in cystic fibrosis [The effect of different exercise modalities applied by tele rehabilitation on functional capacity, oxidative stress and respiratory parameters in cystic fibrosis children]. clinicaltrials.gov/ct2/show/NCT05147285 (first received 7 December 2021).
NCT05173194 {published data only}
-
- NCT05173194. Remotely supervised exercise for adults with cystic fibrosis [Effects of a remotely supervised exercise program on inflammatory markers, muscle strength and lung function in adult patients with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT05173194 (first received 29 December 2021).
NCT05239611 {published data only}
-
- NCT05239611. Feasibility of home-based exercise program for adults with cystic fibrosis [Feasibility of home-based exercise program for adults with cystic fibrosis to improve patient-centered outcomes, including a novel measure of ventilation]. clinicaltrials.gov/ct2/show/NCT05239611 (first received 15 February 2022).
Additional references
ACSM 2017
-
- American College of Sports Medicine. ACSM's guidelines for exercise testing prescription. 10th edition. Philadelphia (PA): Lippincott Williams and Williams, 2017.
Barrett 2021
-
- Barrett S, Begg S, O'Halloran P, Howlett O, Lawrence J, Kingsley M. The effect of behaviour change interventions on changes in physical activity and anthropometrics in ambulatory hospital settings: a systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity 2021;18(1):7. [DOI: 10.1186/s12966-020-01076-6] - DOI - PMC - PubMed
Burgel 2015
-
- Burgel PR, Bellis G, Olesen HV, Viviani L, Zolin A, Blasi F, et al, on behalf of the ERS/ECFS Task Force on the Provision of Care for Adults with Cystic Fibrosis in Europe. Future trends in cystic fibrosis demography in 34 European countries. European Respiratory Journal 2015;46:133-41. - PubMed
Caspersen 1985
Cerny 2013
-
- Cerny F. Exercise and cystic fibrosis (CF) 2.0. Pediatric Exercise Science 2013;25(4):616-23. - PubMed
Cox 2016
-
- Cox NS, Alison JA, Button BM, Wilson JW, Morton JM, Holland AE. Physical activity participation by adults with cystic fibrosis: an observational study. Respirology 2016;21(3):511-8. - PubMed
Cox 2018
Davies 2020
-
- Davies G, Rowbotham NJ, Smith S, Elliot ZC, Gathercole G, Rayner O, et al. Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. Journal of Cystic Fibrosis 2020;19:499-502. - PubMed
Denford 2020
-
- Denford S, Mackintosh KA, McNarry MA, Barker AR, Williams CA, Active Youth Unlimited Group. Promotion of physical activity for adolescents with cystic fibrosis: a qualitative study of UK multi disciplinary cystic fibrosis teams. Physiotherapy 2020;106:111-8. [DOI: 10.1016/j.physio.2019.01.012] - DOI - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Elce 2018
-
- Elce A, Nigro E, Gelzo M, Iacotucci P, Carnovale V, Liguori R, et al. Supervised physical exercise improves clinical, anthropometric and biochemical parameters in adult cystic fibrosis patients: a 2-year evaluation. Clinical Respiratory Journal 2018;12(7):2228-34. - PubMed
Ellis 2010
-
- Ellis PD. The Essential Guide to Effect Sizes: an Introduction to Statistical Power, Meta-analysis and the Interpretation of Research Results. Cambridge (UK): Cambridge University Press, 2010.
Farrell 2008
-
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450-3. - PubMed
Galassetti 2013
-
- Galassetti P, Riddell MC. Exercise and type 1 diabetes (T1DM). Comprehensive Physiology 2013;3(3):1309-36. - PubMed
Gruet 2022
Hebestreit 2001
-
- Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2001;164(3):443-6. - PubMed
Hebestreit 2014
Hebestreit 2019
-
- Hebestreit H, Hulzebos EH, Schneiderman JE, Karila C, Boas SR, Kriemler S, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2019;199(8):987-95. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Hopewell 2009
Howlett 2019
-
- Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Translational Behavioral Medicine 2019;9(1):147-57. - PMC - PubMed
Ioannidis 1998
-
- Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta-analyses and large trials. JAMA 1998;279(14):1089-93. - PubMed
Keogh 2018
MacKenzie 2014
Maltais 2014
-
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;189(9):e15-62. - PMC - PubMed
Middleton 2019
Nixon 1992
-
- Nixon PA, Orenstein DM, Kelsey SF, Doerschuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. New England Journal of Medicine 1992;327(25):1785-8. - PubMed
Nuss 2021
-
- Nuss K, Moore K, Nelson T, Li K. Effects of motivational interviewing and wearable fitness trackers on motivation and physical activity: a systematic review. American Journal of Health Promotion 2021;35(2):226-35. - PubMed
Pianosi 2005
Puppo 2020
-
- Puppo H, Torres-Castro R, Vasconcello-Castillo L, Acosta-Dighero R, Sepúlveda-Cáceres N, Quiroga-Marabolí P, et al. Physical activity in children and adolescents with cystic fibrosis: a systematic review and meta-analysis. Pediatric Pulmonology 2020;5(11):2863-76. - PubMed
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire – Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;136(6):1610-8. - PMC - PubMed
Radtke 2018a
-
- Radtke T, Hebestreit H, Gallati S, Schneiderman JE, Braun J, Stevens D, et al. CFTR genotype and maximal exercise capacity in cystic fibrosis: a cross-sectional study. Annals of the American Thoracic Society 2018;15(2):209-16. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowbotham 2018
Rowbotham 2020
-
- Rowbotham NJ, Smith SJ, Davies G, Daniel T, Elliott ZC, Gathercole K, et al. Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals. Journal of Cystic Fibrosis 2020;19(4):e19-24. - PubMed
Ruf 2009
Ruf 2010
-
- Ruf K, Winkler B, Hebestreit A, Gruber W, Hebestreit H. Risks associated with exercise testing and sports participation in cystic fibrosis. Journal of Cystic Fibrosis 2010;9(5):339-45. - PubMed
Saynor 2013
-
- Saynor ZL, Barker AR, Oades PJ, Williams CA. Reproducibility of maximal cardiopulmonary exercise testing for young cystic fibrosis patients. Journal of Cystic Fibrosis 2013;12(6):644-50. - PubMed
Schneiderman 2014
-
- Schneiderman JE, Wilkes DL, Atenafu EG, Nguyen T, Wells GD, Alarie N, et al. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. European Respiratory Journal 2014;43(3):817-23. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. - PubMed
Scotet 2020
Shelley 2019
-
- Shelley J, Boddy LM, Knowles ZR, Stewart CE, Dawson EA. Physical activity and associations with clinical outcome measures in adults with cystic fibrosis: a systematic review. Journal of Cystic Fibrosis 2019;18(5):590-601. - PubMed
Shephard 1994
-
- Shephard RJ. Aerobic Fitness and Health. Leeds (UK): Human Kinetic Publishers, 1994.
Southern 2007
-
- Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. Journal of Cystic Fibrosis 2007;6 (1):57-65. - PubMed
Stanojevic 2016
-
- Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. Journal of Cystic Fibrosis 2016;15(4):416-23. - PubMed
Tejero García 2011
-
- Tejero García S, Giráldez Sánchez MA, Cejudo P, Quintana Gallego E, Dapena J, García Jiménez R, et al. Bone health, daily physical activity, and exercise tolerance in patients with cystic fibrosis. Chest 2011;140(2):475-81. - PubMed
Tomlinson 2021
van de Weert‐Van Leeuwen 2012
-
- de Weert-Van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, Van der Ent CK, Arets HG. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. European Respiratory Journal 2012;39:893-8. - PubMed
van de Weert‐Van Leeuwen 2013
Wade 2006
Wainwright 2015
Wilkinson 2019
-
- Wilkinson JW, Watson EL, Xenophontos S, Gould DW, Smith AC. The "minimum clinically important difference" in frequently reported objective physical function tests after a 12-week renal rehabilitation exercise intervention in nondialysis chronic kidney disease. American Journal of Physical Medicine & Rehabilitation 2019;98(6):431-7. - PubMed
Wilson 2021
-
- Wilson J, You X, Ellis M, Urquhart DS, Jha L, Duncan M, et al. VO 2max as an exercise tolerance endpoint in people with cystic fibrosis: lessons from a lumacaftor/ivacaftor trial. Journal of Cystic Fibrosis 2021;20(3):499-505. - PubMed
References to other published versions of this review
Bradley 2002
Bradley 2008
Radtke 2015
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

