Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review
- PMID: 35943061
- PMCID: PMC9361430
- DOI: 10.1002/14651858.CD015021
Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review
Abstract
Background: High efficacy in terms of protection from severe COVID-19 has been demonstrated for several SARS-CoV-2 vaccines. However, patients with compromised immune status develop a weaker and less stable immune response to vaccination. Strong immune response may not always translate into clinical benefit, therefore it is important to synthesise evidence on modified schemes and types of vaccination in these population subgroups for guiding health decisions. As the literature on COVID-19 vaccines continues to expand, we aimed to scope the literature on multiple subgroups to subsequently decide on the most relevant research questions to be answered by systematic reviews.
Objectives: To provide an overview of the availability of existing literature on immune response and long-term clinical outcomes after COVID-19 vaccination, and to map this evidence according to the examined populations, specific vaccines, immunity parameters, and their way of determining relevant long-term outcomes and the availability of mapping between immune reactivity and relevant outcomes.
Search methods: We searched the Cochrane COVID-19 Study Register, the Web of Science Core Collection, and the World Health Organization COVID-19 Global literature on coronavirus disease on 6 December 2021. SELECTION CRITERIA: We included studies that published results on immunity outcomes after vaccination with BNT162b2, mRNA-1273, AZD1222, Ad26.COV2.S, Sputnik V or Sputnik Light, BBIBP-CorV, or CoronaVac on predefined vulnerable subgroups such as people with malignancies, transplant recipients, people undergoing renal replacement therapy, and people with immune disorders, as well as pregnant and breastfeeding women, and children. We included studies if they had at least 100 participants (not considering healthy control groups); we excluded case studies and case series.
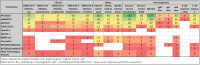
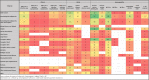
Data collection and analysis: We extracted data independently and in duplicate onto an online data extraction form. Data were represented as tables and as online maps to show the frequency of studies for each item. We mapped the data according to study design, country of participant origin, patient comorbidity subgroup, intervention, outcome domains (clinical, safety, immunogenicity), and outcomes. MAIN RESULTS: Out of 25,452 identified records, 318 studies with a total of more than 5 million participants met our eligibility criteria and were included in the review. Participants were recruited mainly from high-income countries between January 2020 and 31 October 2021 (282/318); the majority of studies included adult participants (297/318). Haematological malignancies were the most commonly examined comorbidity group (N = 54), followed by solid tumours (N = 47), dialysis (N = 48), kidney transplant (N = 43), and rheumatic diseases (N = 28, 17, and 15 for mixed diseases, multiple sclerosis, and inflammatory bowel disease, respectively). Thirty-one studies included pregnant or breastfeeding women. The most commonly administered vaccine was BNT162b2 (N = 283), followed by mRNA-1273 (N = 153), AZD1222 (N = 66), Ad26.COV2.S (N = 42), BBIBP-CorV (N = 15), CoronaVac (N = 14), and Sputnik V (N = 5; no studies were identified for Sputnik Light). Most studies reported outcomes after regular vaccination scheme. The majority of studies focused on immunogenicity outcomes, especially seroconversion based on binding antibody measurements and immunoglobulin G (IgG) titres (N = 179 and 175, respectively). Adverse events and serious adverse events were reported in 126 and 54 studies, whilst SARS-CoV-2 infection irrespective of severity was reported in 80 studies. Mortality due to SARS-CoV-2 infection was reported in 36 studies. Please refer to our evidence gap maps for more detailed information.
Authors' conclusions: Up to 6 December 2021, the majority of studies examined data on mRNA vaccines administered as standard vaccination schemes (two doses approximately four to eight weeks apart) that report on immunogenicity parameters or adverse events. Clinical outcomes were less commonly reported, and if so, were often reported as a secondary outcome observed in seroconversion or immunoglobulin titre studies. As informed by this scoping review, two effectiveness reviews (on haematological malignancies and kidney transplant recipients) are currently being conducted.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Nina Kreuzberger: no relevant interests; staff role at Cochrane Haematology, University Hospital Cologne, Germany; member of the prognosis methods group.
Caroline Hirsch: no relevant interests; Managing Editor at Cochrane Haematology, but was not involved in the editorial process for this review.
Marike Andreas: none known.
Lena Böhm: none known.
Paul Bröckelmann: BeiGene USA, Inc. (grant/contract), Bristol‐Myers Squibb Foundation (grant/contract), Celgene (travel), Takeda Oncology (consultant); consultant for internal medicine fellow in haematology/oncology, Department of Internal Medicine, University Hospital Cologne, Germany.
Veronica Di Cristanziano: no relevant interests; virologist at the Institute of Virology of the University Hospital Cologne, Germany; involved in the study 'Immune responses to SARS‐CoV‐2 infection and vaccination in dialysis patients and kidney transplant recipients'. The study at hand was supported by intramural funds.
Martin Golinski: no relevant interests; anesthesiologist consultant in intensive care medicine, Department of Anesthesiology, University Medicine Center Goettingen, Georg August University, Goettingen, Germany.
Renate Hausinger: no relevant interests; resident, Department of Nephrology at Klinikum rechts der Isar, University Hospital, Munich, Germany.
Verena Kappler: none known.
Berit Lange: Bundesministerium für Bildung und Forschung (grant/contract for MuSPAD seroprevalence study).
Tina Lischetzki: none known.
Sibylle Mellinghoff: Gilead Foundation (travel), Octapharma USA Inc (consultant).
Agata Mikolajewska: no relevant interests; co‐ordination of Section COVRIIN and Work in Office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
Ina Monsef: no relevant interests; Information Specialist, Cochrane Haematology.
Yun Soo Park: none known.
Vanessa Piechotta: none known.
Christoph Schmaderer: none known.
Miriam Stegemann: no relevant interests; medical doctor, Charité Universitätsmedizin Berlin, Germany.
Kanika Vanshylla: patent regarding SARS‐2 neutralising antibodies filed by the University of Cologne (pending); Tober‐Lau P, Gruell H, Vanshylla K, et al. doi:10.3201/eid2805.220271 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF); Vanshylla K, Tober‐Lau P, Gruell H, et al. doi:10.1016/S1473‐3099(22)00135‐9 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF) Deutsche Forschungsgemeinschaft (DFG); Gruell H, Vanshylla K, Tober‐Lau P, et al. doi:10.1038/s41591‐021‐01676‐0 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF) Deutsche Forschungsgemeinschaft (DFG); Mellinghoff SC, Robrecht S, Mayer L, et al. doi:10.1038/s41375‐021‐01500‐1 German Center for Infection Research (DZIF) Mildred Scheel School of Oncology German Cancer Aid; Müller L, Andrée M, Ostermann PN, et al. doi:10.3389/fmed.2021.746644 VIRus ALliance NRW (VIRAL) from the Ministry of Culture and Science NRW Jürgen Manchot Foundation; Tober‐Lau P, Schwarz T, Vanshylla K, et al. doi:10.1016/S2213‐2600(21)00456‐2 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Berlin Institute of Health (BIH) and Berlin University Alliance Deutsche Forschungsgemeinschaft (DFG); Hillus D, Schwarz T, Tober‐Lau P, et al. doi:10.1016/S2213‐2600(21)00357‐X COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung, Laboratory of Experimental Immunology, Institute of Virology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Florencia Weber: no relevant interests; resident of anaesthesiology, Universitätsklinikum Würzburg, Germany.
Stephanie Weibel: no relevant interests; editor with Cochrane Anaesthesia.
Caspar Stephani: no relevant interests; medical doctor on an intensive care unit in Göttingen, Germany.
Nicole Skoetz: no relevant interests; editor with Cochrane Haematology, but was not involved in the editorial process for this review.
Figures
References
Additional references
Abu‐Raddad 2022
Barajas‐Nava 2021
Basta 2022
-
- Basta NE, Moodie EMM on behalf of the VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team. COVID-19 vaccine tracker. covid19.trackvaccines.org/vaccines/ (accessed 20 December 2021).
Brockmann 2022
CDC 2022
-
- Centers for Disease Control and Prevention. Joint statement from HHS Public Health and medical experts on COVID-19 booster shots. www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed 2 February 2022).
Cele 2022
Collier 2021
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed 21 December 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Dejnirattisai 2022
Earle 2021
EMA 2021a
-
- European Medicines Agency. Human regulatory - COVID-19 vaccines. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/cor... (accessed 14 March 2022).
EMA 2021b
-
- European Medicines Agency. EMA recommends Nuvaxovid for authorisation in the EU. www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu (accessed 31 May 2022).
Feikin 2022
-
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg V, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399(10328):924-44. [DOI: 10.1016/S0140-6736(22)00152-0] - DOI - PMC - PubMed
Garcia‐Beltran 2022
Gruell 2022
Hausinger 2022
-
- Hausinger R, Bachmann Q, Crone-Rawe T, Hannane N, Schmaderer C, Kreuzberger N, et al. Efficacy, immunogenicity and harms of SARS-CoV-2 booster vaccination for kidney transplant recipients: a systematic review. osf.io/nsyq4 (accessed prior to 8 July 2022).
Herishanu 2021
Hillus 2021
-
- Hillus D, Schwarz T, Tober-Lau P, Hastor H, Thibeault C, Kasper S, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. Lancet Respiratory Medicine 2021;9(11):1255-65. [DOI: 10.1016/S2213-2600(21)00357-X] - DOI - PMC - PubMed
International Initiative for Impact Evaluation
-
- International Initiative for Impact Evaluation. Evidence gap maps. www.3ieimpact.org/evidence-hub/evidence-gap-maps (accessed 31 May 2022).
Khoury 2021
Krammer 2020
Krause 2021
Kreuzberger 2021
-
- Kreuzberger N, Hirsch C, Andreas M, Böhm L, Bröckelmann PJ, Di Cristanziano V, et al. Immunity after COVID-19 vaccination: protocol for a scoping review. doi.org/10.17605/OSF.IO/HTGB8 (accessed prior to 8 July 2022). [DOI: 10.17605/OSF.IO/HTGB8] - DOI - PMC - PubMed
Lee 2022
Levine‐Tiefenbrun 2022
Ligumsky 2022
Lusvarghi 2021
-
- Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster–elicited serum but evades most convalescent serum and therapeutic antibodies. Science Translational Medicine 2021;14(645):eabn8543. [DOI: 10.1126/scitranslmed.abn8543] - DOI - PMC - PubMed
Mahmoud 2021
Moher 2009
MS Excel [Computer program]
-
- Microsoft Excel. Microsoft Corporation, 2018. Available at office.microsoft.com/excel.
Muecksch 2020
Osmanodja 2022
Pandey 2020
Patel 2021
Perkmann 2021
-
- Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiology Spectrum 2021;9(1):e00247-21. [DOI: 10.1128/Spectrum.00247-21] - DOI - PMC - PubMed
Piechotta 2022
-
- Piechotta V, Mellinghoff SC, Hirsch C, Brinkmann A, Iannizzi C, Kreuzberger N, et al. Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review. Blood Cancer Journal 2022;12(5):86. [DOI: 10.1038/s41408-022-00684-8] - DOI - PMC - PubMed
Public Health Ontario 2021
-
- Ontario Agency for Health Protection and Promotion. COVID-19 real-world vaccine effectiveness – what we know so far. www.publichealthontario.ca/-/media/documents/ncov/covid-wwksf/2021/04/ww... (accessed 16 March 2022).
Ramos 2022
R Core Team [Computer program]
-
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at R-project.org.
Rubio‐Acero 2021
-
- Rubio-Acero R, Castelletti N, Fingerle V, Olbrich L, Bakuli A, Wölfel R, et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infectious Diseases and Therapy 2021;10:1505-18. [DOI: 10.1007/s40121-021-00475-x. Epub] - PMC - PubMed
Schmidt 2021
Schmidt 2022
Schrezenmeier 2022
-
- Schrezenmeier E, Rincon-Arevalo H, Jens A, Stefanski AL, Hammett C, Osmanodja B, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022;7(9):e157836. [DOI: 10.1172/jci.insight.157836] - DOI - PMC - PubMed
Schwarz 2021
Sette 2021
Sholukh 2021
Shrotri 2021
Thakkar 2021
Tober‐Lau 2021
Tricco 2018
Viana 2022
WHO 2020
-
- World Health Organization. Establishment of the WHO international standard and reference panel for anti-SARS-CoV-2 antibody. www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed 31 May 2022).
WHO 2022a
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. www.covid19.who.int (accessed 15 May 2022).
WHO 2022b
-
- World Health Organization. Tracking SARS-CoV-2 variants. www.who.int/activities/tracking-SARS-CoV-2-variants (accessed 31 May 2022). - PubMed
WHO 2022c
-
- World Health Organization. COVAX - Working for global equitable access to COVID-19 vaccines. www.who.int/initiatives/act-accelerator/covax (accessed 15 May 2022).
Wouters 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous