Cerebral metabolic derangements following traumatic brain injury
- PMID: 35943124
- PMCID: PMC9594147
- DOI: 10.1097/ACO.0000000000001183
Cerebral metabolic derangements following traumatic brain injury
Abstract
Purpose of review: Outcome following traumatic brain injury (TBI) remains variable, and derangements in cerebral metabolism are a common finding in patients with poor outcome. This review compares our understanding of cerebral metabolism in health with derangements seen following TBI.
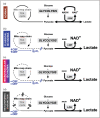
Recent findings: Ischemia is common within the first 24 h of injury and inconsistently detected by bedside monitoring. Metabolic derangements can also result from tissue hypoxia in the absence of ischemic reductions in blood flow due to microvascular ischemia and mitochondrial dysfunction. Glucose delivery across the injured brain is dependent on blood glucose and regional cerebral blood flow, and is an important contributor to derangements in glucose metabolism. Alternative energy substrates such as lactate, ketone bodies and succinate that may support mitochondrial function, and can be utilized when glucose availability is low, have been studied following TBI but require further investigation.
Summary: Mitochondrial dysfunction and the use of alternative energy substrates are potential therapeutic targets, but improved understanding of the causes, impact and significance of metabolic derangements in clinical TBI are needed. Maintaining adequate oxygen and glucose delivery across the injured brain may accelerate the recovery of mitochondrial function and cerebral energy metabolism and remain important management targets.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures

References
-
- Dijkland SA, Helmrich I, Nieboer D, et al. . Outcome prediction after moderate and severe traumatic brain injury: external validation of two established prognostic models in 1742 European patients. J Neurotrauma 2021; 38:1377–1388. - PubMed
-
- Hoffman H, Abi-Aad K, Bunch KM, et al. . Outcomes associated with brain tissue oxygen monitoring in patients with severe traumatic brain injury undergoing intracranial pressure monitoring. J Neurosurg 2021; 135:1799–1806. - PubMed
-
- Guilfoyle MR, Helmy A, Donnelly J, et al. . Characterising the dynamics of cerebral metabolic dysfunction following traumatic brain injury: a microdialysis study in 619 patients. PLoS One 2021; 16:e0260291. - PMC - PubMed
-
Large-scale retrospective study highlighting the prevalence of metabolic injuries shown on cerebral microdialysis and its association with neurological outcomes and various modifiable monitoring variables such as cerebral perfusion pressure, pressure reactivity index, PbtO2 and brain glucose. Threshold values associated with higher lactate/pyruvate ratio for these variables are identified which provides grounds for the selection of specific treatment targets.
-
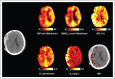
- Launey Y, Fryer TD, Hong YT, et al. . Spatial and temporal pattern of ischemia and abnormal vascular function following traumatic brain injury. JAMA Neurol 2020; 77:339–349. - PMC - PubMed
-
In-depth multitracer oxygen-15 PET study improving our understanding of macrovascular ischemia, both in the perilesional area and away from the area of visible injury. These changes were shown to be often undetectable by jugular bulb oximetry and brain tissue oxygen probes and sometimes present despite normal cerebral perfusion pressure and intracranial pressure.
-
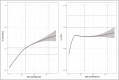
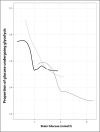
- Hermanides J, Hong YT, Trivedi M, et al. . Metabolic derangements are associated with impaired glucose delivery following traumatic brain injury. Brain 2021; 144:3492–3504. - PMC - PubMed
-
This cohort study used PET imaging to improve our understanding of changes in glucose metabolism following traumatic brain injury (TBI) and its relationship with ischemic brain. It identifies compensating mechanisms (upregulation of K1) to preserve glucose delivery in the setting of reduced cerebral blood flow and reveals targets for both plasma and cerebral glucose.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

