A Dynamic Interplay of Circulating Extracellular Vesicles and Galectin-1 Reprograms Viral Latency during HIV-1 Infection
- PMID: 35943163
- PMCID: PMC9426495
- DOI: 10.1128/mbio.00611-22
A Dynamic Interplay of Circulating Extracellular Vesicles and Galectin-1 Reprograms Viral Latency during HIV-1 Infection
Abstract
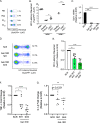
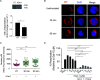
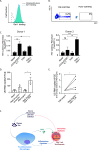
Combined Antiretroviral therapy (cART) suppresses HIV replication but fails to eradicate the virus, which persists in a small pool of long-lived latently infected cells. Immune activation and residual inflammation during cART are considered to contribute to viral persistence. Galectins, a family of β-galactoside-binding proteins, play central roles in host-pathogen interactions and inflammatory responses. Depending on their structure, glycan binding specificities and/or formation of distinct multivalent signaling complexes, different members of this family can complement, synergize, or oppose the function of others. Here, we identify a regulatory circuit, mediated by galectin-1 (Gal-1)-glycan interactions, that promotes reversal of HIV-1 latency in infected T cells. We found elevated levels of circulating Gal-1 in plasma from HIV-1-infected individuals, which correlated both with inflammatory markers and the transcriptional activity of the reservoir, as determined by unspliced-RNA (US-RNA) copy number. Proinflammatory extracellular vesicles (EVs) isolated from the plasma of HIV-infected individuals induced Gal-1 secretion by macrophages. Extracellularly, Gal-1 interacted with latently infected resting primary CD4+ T cells and J-LAT cells in a glycan-dependent manner and reversed HIV latency via activation of the nuclear factor κB (NF-κB). Furthermore, CD4+ T cells isolated from HIV-infected individuals showed increased HIV-1 transcriptional activity when exposed to Gal-1. Thus, by modulating reservoir dynamics, EV-driven Gal-1 secretion by macrophages links inflammation with HIV-1 persistence in cART-treated individuals. IMPORTANCE Antiretroviral therapy has led to a dramatic reduction in HIV-related morbidity and mortality. However, cART does not eradicate the virus, which persists in resting CD4+ T cells as the main viral reservoir, consequently requiring lifelong treatment. A major question is how the functional status of the immune system during antiretroviral therapy determines the activity and size of the viral reservoir. In this study, we identified a central role for galectin-1 (Gal-1), a glycan-binding protein released in response to extracellular vesicles (EVs), in modulating the activity of HIV reservoir, thus shaping chronic immune activation in HIV-infected patients. Our work unveils a central role of Gal-1 in linking chronic immune activation and reservoir dynamics, highlighting new therapeutic opportunities in HIV infection.
Keywords: chronic inflammation; extracellular vesicles; galectin-1; human immunodeficiency virus; viral reservoir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Galectin-9 Mediates HIV Transcription by Inducing TCR-Dependent ERK Signaling.Front Immunol. 2019 Feb 20;10:267. doi: 10.3389/fimmu.2019.00267. eCollection 2019. Front Immunol. 2019. PMID: 30842775 Free PMC article.
-
Extracellular vesicle-mediated intercellular communication in HIV-1 infection and its role in the reservoir maintenance.Cytokine Growth Factor Rev. 2020 Feb;51:40-48. doi: 10.1016/j.cytogfr.2019.12.006. Epub 2019 Dec 27. Cytokine Growth Factor Rev. 2020. PMID: 31926807 Review.
-
Maraviroc Is Associated with Latent HIV-1 Reactivation through NF-κB Activation in Resting CD4+ T Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy.J Virol. 2018 Apr 13;92(9):e01931-17. doi: 10.1128/JVI.01931-17. Print 2018 May 1. J Virol. 2018. PMID: 29444937 Free PMC article.
-
Induction of HIF-1α by HIV-1 Infection in CD4+ T Cells Promotes Viral Replication and Drives Extracellular Vesicle-Mediated Inflammation.mBio. 2018 Sep 11;9(5):e00757-18. doi: 10.1128/mBio.00757-18. mBio. 2018. PMID: 30206166 Free PMC article.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection.Cell Mol Immunol. 2023 Oct;20(10):1101-1113. doi: 10.1038/s41423-023-01074-1. Epub 2023 Aug 15. Cell Mol Immunol. 2023. PMID: 37582971 Free PMC article. Review.
-
The immunosuppressive tuberculosis-associated microenvironment inhibits viral replication and promotes HIV-1 latency in CD4+ T cells.iScience. 2024 Jun 20;27(7):110324. doi: 10.1016/j.isci.2024.110324. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055929 Free PMC article.
-
Exosomes, microvesicles, and other extracellular vesicles-a Keystone Symposia report.Ann N Y Acad Sci. 2023 May;1523(1):24-37. doi: 10.1111/nyas.14974. Epub 2023 Mar 18. Ann N Y Acad Sci. 2023. PMID: 36961472 Free PMC article.
References
-
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sékaly R-P. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi:10.1038/nm.1972. - DOI - PMC - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi:10.1126/science.278.5341.1295. - DOI - PubMed
-
- Chun T-W, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, Sheth PM, Kaul R, Ostrowski M, Moir S, Kovacs C, Fauci AS. 2010. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24:2803–2808. doi:10.1097/QAD.0b013e328340a239. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
