Real-life benefits of intrajejunal levodopa infusion therapy in four patients with the parkinsonian variant of progressive supranuclear palsy: A 1-year follow-up data report
- PMID: 35943202
- PMCID: PMC9480946
- DOI: 10.1002/brb3.2547
Real-life benefits of intrajejunal levodopa infusion therapy in four patients with the parkinsonian variant of progressive supranuclear palsy: A 1-year follow-up data report
Abstract
Background: Progressive supranuclear palsy (PSP) is a progressive neurodegenerative condition presenting with different clinical endophenotypes. The parkinsonian variant of PSP (PSP-P) is characterised by early but fading responsiveness to high-dose levodopa therapy; however, high-dose oral therapy is often associated with intolerance due to dopaminergic side effects and so doses may have to be capped despite clinical benefits. Evidence from animal models and real-life registries suggest far higher doses of levodopa can be tolerated if given in a continuous drug delivery (CDD) manner. We investigated tolerance and possible clinical benefits in patients with PSP-P still responsive to levodopa after initiating CDD in the form of intrajejunal levodopa infusion (IJLI) therapy as part of a compassionate usage program (CU).
Methods: This is an observational clinical data report from the IJLI implementation program undertaken in regional tertiary referral Parkinson's centres in India and at King's College Hospital London, Dubai as part of a CU. Four patients with PSP-P receiving IJLI as a part of a CU underwent evaluations of liver and renal function, motor and nonmotor function, quality of life, sleep dysfunction, fatigue, anxiety and depression, and cognitive impairment at baseline and 6 and 12 months post-IJLI initiation.
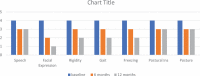
Results: In total, three out of four patients successfully completed 12 months of treatment (6 months in one patient). All four patients showed good tolerability to IJLI even at higher doses (1400 and 1960 mg at 6 and 12 months, respectively) when compared to oral levodopa (812.5 ± 103 levodopa equivalent daily dose [LEDD]) and presented with overall persistent improvements in motor and nonmotor scores and quality-of-life scores at 6 and 12 months post-IJLI. All patients showed improvement in estimated glomerular filtration rate (43.50 ml/min/1.73 m2 to 67.5 ml/min/1.73 m2 and 79.5 ml/min/1.73 m2 at 6 and 12 months, respectively).
Conclusions: IJLI led to persistent beneficial effects on motor and some nonmotor aspects in patients with PSP-P at up to 12 months after treatment with associated improvement in overall renal function.
Keywords: Parkinsonian variant of progressive supranuclear palsy; intrajejunal levodopa-carbidopa infusion; motor and nonmotor symptoms; quality of life.
© 2022 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Antonini, A. , Poewe, W. , Chaudhuri, K. R. , Jech, R. , Pickut, B. , Pirtošek, Z. , Szasz, J. , Valldeoriola, F. , Winkler, C. , Bergmann, L. , Yegin, A. , Onuk, K. , Barch, D. , Odin, P. , & GLORIA Study Co‐investigators (2017). Levodopa‐carbidopa intestinal gel in advanced Parkinson's: Final results of the GLORIA registry. Parkinsonism & Related Disorders, 45, 13–20. 10.1016/j.parkreldis.2017.09.018 - DOI - PubMed
-
- Höglinger, G. U. , Respondek, G. , Stamelou, M. , Kurz, C. , Josephs, K. A. , Lang, A. E. , Mollenhauer, B. , Müller, U. , Nilsson, C. , Whitwell, J. L. , Arzberger, T. , Englund, E. , Gelpi, E. , Giese, A. , Irwin, D. J. , Meissner, W. G. , Pantelyat, A. , Rajput, A. , van Swieten, J. C. , … Litvan, I. , Movement Disorder Society‐endorsed PSP Study Group (2017). Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders, 32, 853–864. 10.1002/mds.26987 - DOI - PMC - PubMed
-
- Metta, V. , Batzu, L. , Leta, V. , Trivedi, D. , Powdleska, A. , Mridula, K. R. , Kukle, P. , Goyal, V. , Borgohain, R. , Chung‐Faye, G. , & Chaudhuri, K. R. (2021). Parkinson's disease: Personalized pathway of care for device‐aided therapies (DAT) and the role of continuous objective monitoring (COM) using wearable sensors. Journal of Personalized Medicine, 11, 680. 10.3390/jpm11070680 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

