Broad-spectrum antiviral activity of Spatholobus suberectus Dunn against SARS-CoV-2, SARS-CoV-1, H5N1, and other enveloped viruses
- PMID: 35943221
- PMCID: PMC9537938
- DOI: 10.1002/ptr.7452
Broad-spectrum antiviral activity of Spatholobus suberectus Dunn against SARS-CoV-2, SARS-CoV-1, H5N1, and other enveloped viruses
Abstract
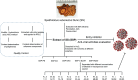
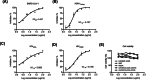
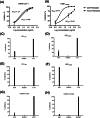
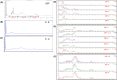
The current COVID-19 pandemic caused by SARS-Cov-2 is responsible for more than 6 million deaths globally. The development of broad-spectrum and cost-effective antivirals is urgently needed. Medicinal plants are renowned as a complementary approach in which antiviral natural products have been established as safe and effective drugs. Here, we report that the percolation extract of Spatholobus suberectus Dunn (SSP) is a broad-spectrum viral entry inhibitor against SARS-CoV-1/2 and other enveloped viruses. The viral inhibitory activities of the SSP were evaluated by using pseudotyped SARS-CoV-1 and 2, HIV-1ADA and HXB2 , and H5N1. SSP effectively inhibited viral entry and with EC50 values ranging from 3.6 to 5.1 μg/ml. Pre-treatment of pseudovirus or target cells with SSP showed consistent inhibitory activities with the respective EC50 value of 2.3 or 2.1 μg/ml. SSP blocked both SARS-CoV-2 spike glycoprotein and the host ACE2 receptor. In vivo studies indicated that there was no abnormal toxicity and behavior in long-term SSP treatment. Based on these findings, we concluded that SSP has the potential to be developed as a drug candidate for preventing and treating COVID-19 and other emerging enveloped viruses.
Keywords: H5N1; HIV-1; SARS-CoVs; Spatholobus suberectus Dunn; antivirals.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
Extraction technology, fractions and their pharmacological activities of SSP is patented by authors: Patient No.CN102462730B, No.CN102579425A, No.IP00939(HKU), No.63/319,940(HKU). The authors declare no others conflicts of interest concerning this work.
Figures



Similar articles
-
The use of Pseudotyped Coronaviruses for the Screening of Entry Inhibitors: Green Tea Extract Inhibits the Entry of SARS-CoV-1, MERSCoV, and SARS-CoV-2 by Blocking Receptor-spike Interaction.Curr Pharm Biotechnol. 2022;23(8):1118-1129. doi: 10.2174/1389201022666210810111716. Curr Pharm Biotechnol. 2022. PMID: 34375189
-
Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses.Peptides. 2011 Jul;32(7):1518-25. doi: 10.1016/j.peptides.2011.05.015. Epub 2011 May 19. Peptides. 2011. PMID: 21620914 Free PMC article.
-
Picolinic acid is a broad-spectrum inhibitor of enveloped virus entry that restricts SARS-CoV-2 and influenza A virus in vivo.Cell Rep Med. 2023 Aug 15;4(8):101127. doi: 10.1016/j.xcrm.2023.101127. Epub 2023 Jul 17. Cell Rep Med. 2023. PMID: 37463584 Free PMC article.
-
Comprehensive Analysis of SARS-COV-2 Drug Targets and Pharmacological Aspects in Treating the COVID-19.Curr Mol Pharmacol. 2022;15(2):393-417. doi: 10.2174/1874467214666210811120635. Curr Mol Pharmacol. 2022. PMID: 34382513 Review.
-
Potential inhibitors of SARS-CoV-2: recent advances.J Drug Target. 2021 Apr;29(4):349-364. doi: 10.1080/1061186X.2020.1853736. Epub 2020 Dec 3. J Drug Target. 2021. PMID: 33210953 Review.
Cited by
-
A Comprehensive Update of Various Attempts by Medicinal Chemists to Combat COVID-19 through Natural Products.Molecules. 2023 Jun 20;28(12):4860. doi: 10.3390/molecules28124860. Molecules. 2023. PMID: 37375415 Free PMC article. Review.
-
Spatholobus suberectus inhibits lipogenesis and tumorigenesis in triple-negative breast cancer via activation of AMPK-ACC and K-Ras-ERK signaling pathway.J Tradit Complement Med. 2023 Sep 13;13(6):623-638. doi: 10.1016/j.jtcme.2023.09.002. eCollection 2023 Nov. J Tradit Complement Med. 2023. PMID: 38020549 Free PMC article.
-
Pseudovirus-Based Systems for Screening Natural Antiviral Agents: A Comprehensive Review.Int J Mol Sci. 2024 May 10;25(10):5188. doi: 10.3390/ijms25105188. Int J Mol Sci. 2024. PMID: 38791226 Free PMC article. Review.
-
Identification of diphenylurea derivatives as novel endocytosis inhibitors that demonstrate broad-spectrum activity against SARS-CoV-2 and influenza A virus both in vitro and in vivo.PLoS Pathog. 2023 May 1;19(5):e1011358. doi: 10.1371/journal.ppat.1011358. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37126530 Free PMC article.
-
Identification of medicinal plant-based phytochemicals as a potential inhibitor for SARS-CoV-2 main protease (Mpro) using molecular docking and deep learning methods.Comput Biol Med. 2023 May;157:106785. doi: 10.1016/j.compbiomed.2023.106785. Epub 2023 Mar 11. Comput Biol Med. 2023. PMID: 36931201 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

