Longitudinal Changes in Campylobacter and the Litter Microbiome throughout the Broiler Production Cycle
- PMID: 35943254
- PMCID: PMC9469715
- DOI: 10.1128/aem.00667-22
Longitudinal Changes in Campylobacter and the Litter Microbiome throughout the Broiler Production Cycle
Abstract
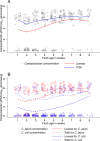
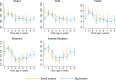
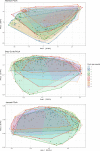
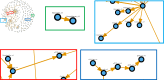
Broiler chickens are an important source of Campylobacter to humans and become colonized on the farm, but the role of the litter in the ecology of Campylobacter is still not clear. The aim of this study was to examine the relationship between Campylobacter and the changes in the litter microbiome throughout the broiler production cycle. Twenty-six commercial broiler flocks representing two production types (small and big broilers) were followed from 1 to 2 weeks after placement to the end of the production cycle. Composite litter samples from the broiler chicken house were collected weekly. Litter DNA was extracted and used for Campylobacter jejuni and Campylobacter coli qPCR as well as for 16S rRNA gene V4 region sequencing. Campylobacter jejuni concentration in litter significantly differed by production type and flock age. Campylobacter jejuni concentration in litter from big broilers was 2.4 log10 units higher, on average, than that of small broilers at 3 weeks of age. Sixteen amplicon sequence variants (ASVs) differentially abundant over time were detected in both production types. A negative correlation of Campylobacter with Bogoriella and Pseudogracilibacillus was observed in the litter microbiome network at 6 weeks of flock age. Dynamic Bayesian networks provided evidence of negative associations between Campylobacter and two bacterial genera, Ornithinibacillus and Oceanobacillus, at 2 and 4 weeks of flock age, respectively. In conclusion, dynamic associations between Campylobacter and the litter microbiome were observed during grow-out, suggesting a potential role of the litter microbiome in the ecology of Campylobacter colonization and persistence on farm. IMPORTANCE This study interrogated the longitudinal association between Campylobacter and broiler litter microbiome in commercial broiler flocks. The results of this investigation highlighted differences in Campylobacter dynamics in the litter throughout the broiler production cycle and between small and big broilers. Besides documenting the changing nature of the microbial networks in broiler litter during grow-out, we detected bacterial genera (Oceanobacillus and Ornithinibacillus) negatively associated with Campylobacter abundance and concentration in litter via the Bayesian network framework. These bacteria should be investigated as possible antagonists to Campylobacter colonization of the broiler environment.
Keywords: Campylobacter; broiler chickens; cohort; litter; microbiome; network.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Association of Broiler Litter Microbiome Composition and Campylobacter Isolation.Front Vet Sci. 2021 May 24;8:654927. doi: 10.3389/fvets.2021.654927. eCollection 2021. Front Vet Sci. 2021. PMID: 34109233 Free PMC article.
-
A Study on Campylobacter jejuni and Campylobacter coli through Commercial Broiler Production Chains in Thailand: Antimicrobial Resistance, the Characterization of DNA Gyrase Subunit A Mutation, and Genetic Diversity by Flagellin A Gene Restriction Fragment Length Polymorphism.Avian Dis. 2017 Jun;61(2):186-197. doi: 10.1637/11546-120116-Reg.1. Avian Dis. 2017. PMID: 28665716
-
Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection.Front Cell Infect Microbiol. 2016 Nov 22;6:154. doi: 10.3389/fcimb.2016.00154. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27921008 Free PMC article.
-
Poultry as a host for the zoonotic pathogen Campylobacter jejuni.Vector Borne Zoonotic Dis. 2012 Feb;12(2):89-98. doi: 10.1089/vbz.2011.0676. Epub 2011 Dec 1. Vector Borne Zoonotic Dis. 2012. PMID: 22133236 Review.
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
Cited by
-
Virulence factors and antimicrobial resistance profiles of Campylobacter isolates recovered from consecutively reused broiler litter.Microbiol Spectr. 2023 Dec 12;11(6):e0323623. doi: 10.1128/spectrum.03236-23. Epub 2023 Oct 26. Microbiol Spectr. 2023. PMID: 37882583 Free PMC article.
-
Deciphering the Association between Campylobacter Colonization and Microbiota Composition in the Intestine of Commercial Broilers.Microorganisms. 2023 Jun 30;11(7):1724. doi: 10.3390/microorganisms11071724. Microorganisms. 2023. PMID: 37512896 Free PMC article.
-
Temporal dynamics of fecal microbiota community succession in broiler chickens, calves, and piglets under aerobic exposure.Microbiol Spectr. 2024 Jun 4;12(6):e0408423. doi: 10.1128/spectrum.04084-23. Epub 2024 May 8. Microbiol Spectr. 2024. PMID: 38717193 Free PMC article.
-
Peeling back the many layers of competitive exclusion.Front Microbiol. 2024 Mar 21;15:1342887. doi: 10.3389/fmicb.2024.1342887. eCollection 2024. Front Microbiol. 2024. PMID: 38591029 Free PMC article.
-
Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms.Antibiotics (Basel). 2023 Sep 19;12(9):1461. doi: 10.3390/antibiotics12091461. Antibiotics (Basel). 2023. PMID: 37760756 Free PMC article.
References
-
- Mughini-Gras L, Pijnacker R, Coipan C, Mulder AC, Fernandes Veludo A, de Rijk S, van Hoek A, Buij R, Muskens G, Koene M, Veldman K, Duim B, van der Graaf-van Bloois L, van der Weijden C, Kuiling S, Verbruggen A, van der Giessen J, Opsteegh M, van der Voort M, Castelijn GAA, Schets FM, Blaak H, Wagenaar JA, Zomer AL, Franz E. 2021. Sources and transmission routes of campylobacteriosis: A combined analysis of genome and exposure data. J Infect 82:216–226. 10.1016/j.jinf.2020.09.039. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

