Oral or Parenteral Methotrexate for the Treatment of Polyarticular Juvenile Idiopathic Arthritis
- PMID: 35943454
- PMCID: PMC10089132
- DOI: 10.5152/eurjrheum.2022.21090
Oral or Parenteral Methotrexate for the Treatment of Polyarticular Juvenile Idiopathic Arthritis
Abstract
Objective: Subcutaneous methotrexate injections are considered to be more effective or work faster than oral methotrexate. Therefore, the extent and the kinetics of response were analyzed in juvenile idiopathic arthritis patients treated with oral versus subcutaneous methotrexate.
Methods: The BIKER databank was searched for biologics-naive juvenile idiopathic arthritis patients treated with methotrexate as initial treatment. The Juvenile Arthritis Disease Activity Score-10 defini- tion of remission and the pediatric American College of Rheumatology's response parameters were utilized as outcome criteria.
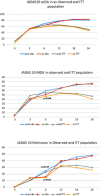
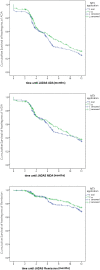
Result: A total of 410 polyarticular juvenile idiopathic arthritis patients receiving oral methotrexate were compared to 384 patients receiving subcutaneous methotrexate. Rheumatoid factor-negative polyarthritis was the most common juvenile idiopathic arthritis category (50%/51%) in this cohort followed by extended oligoarthritis (27%/26%), polyarticular psoriatic arthritis (18%/16%), and few had rheumatoid factor-positive polyarthritis (5%/8%). The oral cohort's disease duration (2.3 ± 3.0 vs. 1.9 ± 2.7) was significantly longer (P=.04), although their age at onset and baseline were similar. Furthermore, at baseline, disease activity (Juvenile Arthritis Disease Activity Score-10 16.5 ± 7.2 vs. 14.7 ± 8.2; P = .001 due to a higher active joint count 9.0 ± 10.1 vs. 7.4 ± 7.7; P = .011) was higher in the subcutaneous cohort. The weekly methotrexate doses were comparable with 13.6 ± 5.4 mg/m2 and 13.3 ± 4.5 mg/m2, respectively. With oral/subcutaneous methotrexate, a pediatric American College of Rheumatology's 90 was achieved in 98(38.3%)/128(40.4%), while 96(38.1 %)/75(40.1%) attained Juvenile Arthritis Disease Activity Score remission after 12 months of therapy. There was no difference in the early kinetics of response according to Kaplan-Meyer analysis. Adverse events including nausea, vomiting, and increased transaminases were considerably more common after methotrexate subcutaneous administration than after oral treatment.
Conclusion: In terms of effectiveness, but not safety, our retrospective analysis found some advan- tages of subcutaneous methotrexate. Adverse effects limit treatment continuance and thus must be considered a disadvantage. Furthermore, oral methotrexate eliminates the need for injections, which is especially essential for younger children. Controlled, randomized prospective trials in children and juvenile patients are necessary for definitive recommendations for the subcutaneous route of admin- istration of methotrexate therapy.
Figures

Similar articles
-
Outcome of children with oligoarticular juvenile idiopathic arthritis compared to polyarthritis on methotrexate- data of the German BIKER registry.Pediatr Rheumatol Online J. 2021 Mar 22;19(1):41. doi: 10.1186/s12969-021-00522-4. Pediatr Rheumatol Online J. 2021. PMID: 33752685 Free PMC article.
-
Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry.Arthritis Care Res (Hoboken). 2012 Sep;64(9):1349-56. doi: 10.1002/acr.21697. Arthritis Care Res (Hoboken). 2012. PMID: 22649024
-
Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial.Lancet. 2021 Nov 27;398(10315):1984-1996. doi: 10.1016/S0140-6736(21)01255-1. Epub 2021 Nov 9. Lancet. 2021. PMID: 34767764 Clinical Trial.
-
Etanercept: an updated review of its use in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis.Drugs. 2002;62(17):2493-537. doi: 10.2165/00003495-200262170-00013. Drugs. 2002. PMID: 12421111 Review.
-
Spotlight on etanercept in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis.BioDrugs. 2003;17(2):139-45. doi: 10.2165/00063030-200317020-00006. BioDrugs. 2003. PMID: 12641492 Review.
Cited by
-
Evolution of treatment options for juvenile idiopathic arthritis.World J Orthop. 2024 Sep 18;15(9):831-835. doi: 10.5312/wjo.v15.i9.831. eCollection 2024 Sep 18. World J Orthop. 2024. PMID: 39318493 Free PMC article.
-
Treatment of non-systemic juvenile idiopathic arthritis.Nat Rev Rheumatol. 2024 Mar;20(3):170-181. doi: 10.1038/s41584-024-01079-8. Epub 2024 Feb 6. Nat Rev Rheumatol. 2024. PMID: 38321298 Review.
-
Methotrexate Intolerance in Juvenile Idiopathic Arthritis: Definition, Risks, and Management.Paediatr Drugs. 2024 Sep;26(5):479-498. doi: 10.1007/s40272-024-00643-9. Epub 2024 Jul 24. Paediatr Drugs. 2024. PMID: 39044097 Free PMC article. Review.
-
Adherence to low-dose methotrexate in children with juvenile idiopathic arthritis using a sensitive methotrexate assay.Pediatr Rheumatol Online J. 2024 May 7;22(1):52. doi: 10.1186/s12969-024-00988-y. Pediatr Rheumatol Online J. 2024. PMID: 38715014 Free PMC article.
References
-
- Giannini EH, Brewer EJ, Kuzmina N.et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and the cooperative children’s Study Group. N Engl J Med. 1992;326(16):1043 1049. 10.1056/NEJM199204163261602) - DOI - PubMed
-
- Gutiérrez-Suárez R, Burgos-Vargas R. The use of methotrexate in children with rheumatic diseases. Clin Exp Rheumatol. 2010;28(5):S122 S127. - PubMed
LinkOut - more resources
Full Text Sources
