Association of Administration of Surfactant Using Less Invasive Methods With Outcomes in Extremely Preterm Infants Less Than 27 Weeks of Gestation
- PMID: 35943742
- PMCID: PMC9364126
- DOI: 10.1001/jamanetworkopen.2022.25810
Association of Administration of Surfactant Using Less Invasive Methods With Outcomes in Extremely Preterm Infants Less Than 27 Weeks of Gestation
Erratum in
-
Errors in a Figure.JAMA Netw Open. 2023 Apr 3;6(4):e2312619. doi: 10.1001/jamanetworkopen.2023.12619. JAMA Netw Open. 2023. PMID: 37083673 Free PMC article. No abstract available.
Abstract
Importance: The inclusion of less invasive surfactant administration (LISA) in the care of preterm infants has been found to be beneficial for respiratory outcomes. Recently, the OPTIMIST trial found higher mortality rates in the subgroup of infants born at 25 to 26 weeks' gestational age (GA) who received surfactant treatment while spontaneously breathing.
Objective: To analyze outcomes among LISA-exposed, highly vulnerable babies born at less than 27 weeks' GA within the large-scale observational cohort of the German Neonatal Network.
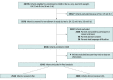
Design, setting, and participants: In this cohort study of data from 68 tertiary level neonatal intensive care units in Germany of infants born between 22 weeks 0 days to 26 weeks 6 days of gestation between April 1, 2009, and December 31, 2020, short-term outcomes among infants receiving LISA vs infants not receiving LISA were compared.
Exposure: Use of LISA within the first 72 hours of life.
Main outcomes and measures: The main outcomes were rates of LISA use, use of mechanical ventilation within the first 72 hours (considered failure of LISA), and association of LISA with outcomes, including death from all causes, bronchopulmonary dysplasia (BPD), death and BPD combined, pneumothorax, retinopathy of prematurity, intracerebral hemorrhage, and periventricular leukomalacia. To address potential confounding factors, multivariate logistic regression models were used.
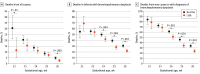
Results: A total of 6542 infants (3030 [46.3%] female and 3512 [53.7%] male; mean [SD] GA, 25.3 (1.1) weeks; mean [SD] birth weight, 715 [180] g) were analyzed; 2534 infants (38.7%) received LISA, which was most frequently given quasi-prophylactically during delivery room management. Among the infants who received LISA, 1357 (53.6%) did not require mechanical ventilation in the first 72 hours compared with 331 infants (8.3%) of 4008 who did not receive LISA. In a multivariate logistic regression model that adjusted for GA, small-for-GA status, sex, multiple birth, inborn status, antenatal steroid use, and maximum fraction of inspired oxygen in the first 12 hours of life, LISA was associated with reduced risks of all-cause death (odds ratio [OR], 0.74; 95% CI, 0.61-0.90; P = .002), BPD (OR, 0.69; 95% CI, 0.62-0.78; P < .001), and BPD or death (OR, 0.64; 95% CI, 0.57-0.72; P < .001) compared with infants without LISA exposure.
Conclusions and relevance: The results of this long-term multicenter cohort study suggest that LISA may be associated with reduced risks of adverse outcomes in extremely preterm infants.
Conflict of interest statement
Figures
References
-
- Göpel W, Kribs A, Ziegler A, et al. ; German Neonatal Network . Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378(9803):1627-1634. doi:10.1016/S0140-6736(11)60986-0 - DOI - PubMed
-
- Dargaville PA, Kamlin COF, Orsini F, et al. ; OPTIMIST-A Trial Investigators . Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: The OPTIMIST—a randomized clinical trial. JAMA. 2021;326(24):2478-2487. doi:10.1001/jama.2021.21892 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

