Rare metabolic disease mimicking COL4A1/COL4A2 fetal brain phenotype
- PMID: 35943828
- PMCID: PMC10695434
- DOI: 10.1002/uog.26046
Rare metabolic disease mimicking COL4A1/COL4A2 fetal brain phenotype
Abstract
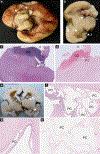
Pathogenic variants of collagen type IV alpha 1 and 2 (COL4A1/COL4A2) genes cause various phenotypic anomalies, including intracerebral hemorrhage and a wide spectrum of developmental anomalies. Only 20% of fetuses referred for COL4A1/COL4A2 molecular screening (fetuses with a suspected intracerebral hemorrhage) carry a pathogenic variant in these genes, raising questions regarding the causative anomaly in the remaining 80% of these fetuses. We examined, following termination of pregnancy or in-utero fetal death, a series of 113 unrelated fetuses referred for COL4A1/COL4A2 molecular screening, in which targeted sequencing was negative. Using exome sequencing data and a gene-based collapsing test, we searched for enrichment of rare qualifying variants in our fetal cohort in comparison to the Genome Aggregation Database (gnomAD) control cohort (n = 71 702). Qualifying variants in pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1) were overrepresented in our cohort, reaching genome-wide significance (P = 2.11 × 10-7 ). Heterozygous PDHA1 loss-of-function variants were identified in three female fetuses. Among these three cases, we observed microcephaly, ventriculomegaly, germinolytic pseudocysts, agenesis/dysgenesis of the corpus callosum and white-matter anomalies that initially suggested cerebral hypoxic-ischemic and hemorrhagic lesions. However, a careful a-posteriori reanalysis of imaging and postmortem data showed that the observed lesions were also consistent with those observed in fetuses carrying PDHA1 pathogenic variants, strongly suggesting that these two phenotypes may overlap. Exome sequencing should therefore be performed in fetuses referred for COL4A1/COL4A2 molecular screening which are screen-negative, with particular attention paid to the PDHA1 gene. © 2022 International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: COL4A1/COL4A2; PDHA1; corpus callosal dysgenesis; exome sequencing; fetal intracerebral hemorrhage; ventriculomegaly.
© 2022 International Society of Ultrasound in Obstetrics and Gynecology.
Figures


References
-
- Imbard A, Boutron A, Vequaud C, Zater M, de Lonlay P, de Baulny HO, Barnerias C, Miné M, Marsac C, Saudubray JM, Brivet M. Molecular characterization of 82 patients with pyruvate dehydrogenase complex deficiency. Structural implications of novel amino acid substitutions in E1 protein. Mol Genet Metab 2011; 104: 507–516. - PubMed
-
- Ganetzky R, McCormick EM, Falk MJ. Primary Pyruvate Dehydrogenase Complex Deficiency Overview. 2021. Jun 17. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A (eds). GeneReviews® [Internet]. University of Washington: Seattle, WA, 1993–2022. https://www.ncbi.nlm.nih.gov/books/NBK571223/
-
- DeBrosse SD, Okajima K, Zhang S, Nakouzi G, Schmotzer CL, Lusk-Kopp M, Frohnapfel MB, Grahame G, Kerr DS. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: lack of correlation with genotype. Mol Genet Metab 2012; 107: 394–402. - PubMed
-
- Savvidou A, Ivarsson L, Naess K, Eklund EA, Lundgren J, Dahlin M, Frithiof D, Sofou K, Darin N. Novel imaging findings in pyruvate dehydrogenase complex (PDHc) deficiency - Results from a nationwide population-based study. J Inherit Metab Dis 2022; 45: 248–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

